A sensory cell diversifies its output by varying Ca2+ influx-release coupling among active zones
- PMID: 33346936
- PMCID: PMC7917556
- DOI: 10.15252/embj.2020106010
A sensory cell diversifies its output by varying Ca2+ influx-release coupling among active zones
Abstract
The cochlea encodes sound pressures varying over six orders of magnitude by collective operation of functionally diverse spiral ganglion neurons (SGNs). The mechanisms enabling this functional diversity remain elusive. Here, we asked whether the sound intensity information, contained in the receptor potential of the presynaptic inner hair cell (IHC), is fractionated via heterogeneous synapses. We studied the transfer function of individual IHC synapses by combining patch-clamp recordings with dual-color Rhod-FF and iGluSnFR imaging of presynaptic Ca2+ signals and glutamate release. Synapses differed in the voltage dependence of release: Those residing at the IHC' pillar side activated at more hyperpolarized potentials and typically showed tight control of release by few Ca2+ channels. We conclude that heterogeneity of voltage dependence and release site coupling of Ca2+ channels among the synapses varies synaptic transfer within individual IHCs and, thereby, likely contributes to the functional diversity of SGNs. The mechanism reported here might serve sensory cells and neurons more generally to diversify signaling even in close-by synapses.
Keywords: calcium channel; exocytosis; nanodomain; synaptic heterogeneity; wide dynamic range coding.
© 2020 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

- A
Maximum intensity projections of the organ of Corti from the right ear of a P17 mouse injected at P6 with 1–2 µl of AAV9.hSyn.iGluSnFR suspension, immunolabeled for iGluSnFR (GFP), IHCs, OHCs, and SGNs (parvalbumin) and synaptic ribbons and nucleus (CtBP2/RIBEYE). Close‐up (right panel) highlights IHCs innervated by several iGluSnFR‐expressing SGN boutons. Step sizes are 0.6 (left) and 0.5 µm (right), respectively. (Scale bars: 10 µm).
- B
Simultaneous perforated patch‐clamp and imaging of IHCs (1.3 mM [Ca2+]e). IHCs were patch‐clamped from the pillar side, with a patch pipette containing TAMRA‐conjugated CtBP2‐binding peptide. Toward the end of the recording, the membrane patch was ruptured to fill the IHC with the peptide, which stains synaptic ribbons and nucleus. (Left) Two exemplary confocal sections of IHCs showing baseline fluorescence of iGluSnFR‐expressing afferent boutons from both modiolar (IHC1) and pillar (IHC2) side of the cell. Glutamate release from IHCs was evoked upon step depolarizations and detected as fluorescence change (ΔF) of iGluSnFR signal located on the SGN membrane (See (B′)). (Right) Overlaid images of the IHC1 and IHC2, displaying the boutons (iGluSnFR) and the synaptic ribbons (CtBP2), after the recording. (*: transduced afferent boutons, >: synaptic ribbons; Scale bar: 2 µm; see Appendix Fig S2 for the iGluSnFR‐ROI detection routine).
- B′
The stimulus protocol of the example IHCs from (B), displaying the voltage stimulation (Vstim), whole‐cell Ca2+ influx (ICa) and single‐synapse iGluSnFR responses. IHCs were stimulated by 10‐ms‐long step depolarizations to −22 mV from the holding potential of −87 mV (1.3 mM [Ca2+]e), and iGluSnFR fluorescence was recorded at 50 Hz.
- C
Average ΔF/F0 iGluSnFR traces in response to 10‐ms‐long step depolarizations from the holding potential (−87 mV) to a voltage within the physiologically relevant range of receptor potentials: from −62 to −22 mV (applied in pseudo‐randomized order, step‐size 5 mV, perforated patch‐clamp, 1.3 mM [Ca2+]e, n = 29 boutons, N = 11 IHCs from nine mice). Shaded areas show ± SD.
- D
The voltage threshold of glutamate release was low and variable (−47.11 ± 4.09 mV, mean ± SD). The area under the curve of the iGluSnFR signal (top; AUC(ΔF/F0)10ms) from (C) and corresponding whole‐cell QCa (bottom; mean ± SD). Detectable signals were defined here if the peak iGluSnFR signal was two times higher than baseline SD (depicted with *). All synapses had detectable signals in response to depolarizations ≥ −42 mV. (See also Figs EV1 and EV2).

- A
Exemplary single‐synapse iGluSnFR signal in response to repetitive 10‐ms‐long step depolarizations to −23 mV from the holding potential (−87 mV) as Ca2+ channel blocker Zn2+ (1 mM) is perfused in and out of the recording chamber. The temporal sequence of the recordings is encoded by color; darker colors indicate earlier time points as in panel (B) (top).
- B
The time course of the peak iGluSnFR response (top; max(ΔF/F0)10ms) from (A) and corresponding whole‐cell peak Ca2+ current (bottom; perforated patch, 1.3 mM [Ca2+]e). The whole‐cell Ca2+ influx decreases with the perfusion of Zn2+.
- C
(top) ABR waveforms of P29 WT mice, injected with AAV9.hSyn.iGluSnFR virus at P6, were recorded in response to 80 dB clicks (mean ± SEM, three animals). The non‐injected ear was used as a control. (bottom) ABR thresholds of the injected ear and the non‐injected control were comparable. A statistical test was not applied due to the small sample size. The presence of iGluSnFR expression was confirmed by immunostainings after ABR recordings.
- D–G
iGluSnFR signal as a readout of glutamate release increases with stimulus duration along with the IHC's capacitance change (ΔCm). (D) Average responses of iGluSnFR (top), whole‐cell Cm (middle), and Ca2+ currents (bottom) upon step depolarizations to −23 mV from the holding potential of −87 mV for durations from 2 to 100 ms (color coded). Recordings were done in organs of Corti of P15–19 WT mice injected with AAV9.hSyn.iGluSnFR virus (perforated patch‐clamp, 1.3 mM [Ca2+]e, n = 31 boutons, N = 10 IHCs from eight mice). (E) The peak and the AUC of iGluSnFR signal, corresponding whole‐cell ΔCm, and QCa plotted as a function of depolarization duration (mean ± SD). (F, G) The relation of whole‐cell ΔCm and the peak (F) or the AUC (G) of the iGluSnFR signal (mean ± SD, n = 31 boutons, N = 10 IHCs from 8 mice). Both the peak and the AUC of iGluSnFR response correlate with the whole‐cell ΔCm (Pearson's r = 0.63, P < 0.0001 and r = 0.66, P < 0.0001, Student's t‐test, respectively). Depolarization duration is color coded, and the black outlined circles, indicating the means, darken with increasing depolarization duration.
- H
Quantification of exocytosis by whole‐cell ΔCm and single‐synapse iGluSnFR‐AUC. (See Materials and Methods).
- I
The kinetics of iGluSnFR signal. Average iGluSnFR responses (as shown in panel (D) top). Black lines indicate the results of fitting to the average traces per depolarization duration (see Materials and Methods). Time to peak and the decay time constants are obtained from these fits and depicted with the color codes of the depolarization durations.
Exemplary single‐synapse iGluSnFR signal in response to 10‐ms‐long step depolarizations from the holding potential of −87 mV to a voltage within the physiologically relevant range of receptor potentials: from −22 to −62 mV in 5 mV increments, same protocol as in Fig 1C and D.
The peak iGluSnFR fluorescence (top; max(ΔF/F0)10ms) from (A) and corresponding whole‐cell Ca2+ current (bottom; perforated patch‐clamp, 1.3 mM [Ca2+]e). Glutamate release can be detected at −42 mV. The voltage range is color coded: Lighter points indicate more positive potentials.
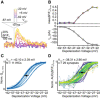
Normalized whole‐cell QCa, calculated in response to 10‐ms‐long step depolarizations, is plotted as a function of depolarization voltage (perforated patch‐clamp, 1.3 mM [Ca2+]e). Individual IHCs are color coded in shades of blue (mean ± SD, N = 11 IHCs from nine mice). A Boltzmann function was fitted to estimate the V10 and V1/2.
Normalized iGluSnFR‐AUC, in response to 10‐ms‐long step depolarizations, same experiments as in (A) (mean ± SD, n = 29 synapses; individual synapses are color coded). A Boltzmann function was fitted to estimate the V10 and V1/2.
Varying the voltage in the hyperpolarized range primarily varies the open‐channel number. RRP release was probed by 10‐ms‐long step depolarizations from the holding potential (−87 mV) to −62 to −22 mV in 5 mV steps applied in pseudo‐randomized order.
The normalized AUC(ΔF/F0)10ms is plotted versus QCa (n = 29 boutons, N = 11 IHCs from nine mice): A power function was fitted before an obvious saturation of the RRP release, and a near‐linear relation was observed (m = 1.55).
Histogram showing the distribution of m from individual fits per synapse before an obvious saturation of the RRP release (mavg = 1.71 ± 0.58). Only those fits with an R 2 value higher than 0.7 were used for further analysis (n = 29 boutons). The rug plot under the histogram displays individual data points. Every depolarization step was repeated at least two times per synapse, and the average was taken.
Manipulation of the single Ca2+ channel current by Zn2+ perfusion. Note that Zn2+ acts as a rapid (microsecond) flicker blocker of Ca2+ channels (Winegar & Lansman, 1990), which, within the limits of IHC exocytosis kinetics (delay ~ 1 ms) (Beutner et al, 2001), is expected to reduce the fusogenic Ca2+ signal at the SV release site (Brandt et al, 2005). Therefore, this manipulation is expected to reveal the supralinear intrinsic Ca2+ dependence of release. We evoked RRP exocytosis by repetitive 10‐ms‐long step depolarizations to −22 mV, while slowly perfusing 1 mM Zn2+ into the recording chamber.
Normalized AUC(ΔF/F0)10 ms is plotted versus QCa upon Zn2+ manipulation (n = 24 boutons, N = 10 IHCs from 10 mice). A power function was fitted before an obvious saturation of RRP release (normalized QCa < 0.7), and a supralinear relation was observed (m = 2.56).
Histogram showing the distribution of m from individual fits synapse before an obvious saturation was observed for a given synapse (m average = 2.50 ± 1.03, n = 21 boutons with an R 2 of fit > 0.7). The rug plot under the histogram displays the individual data points.

- A
IHCs were patch‐clamped in ruptured‐patch configuration with 800 μM Rhod‐FF in the patch pipette, and simultaneously imaged for Ca2+ signals or glutamate release by spinning disk microscopy.
- B
Mean ΔF images of Rhod‐FF and iGluSnFR signals in response to a voltage ramp and a 50‐ms‐long step depolarization, respectively. The synapse marked with * on the overlaid image is further analyzed in the following panels. (P: pillar side, M: modiolar side; Scale bar: 2 μm)
- C, D
Voltage command (top), corresponding whole‐cell Ca2+ influx (middle) and the functional fluorescence responses (bottom; band‐stop filtered at 33.3 Hz) from Rhod‐FF and iGluSnFR, respectively. A modified Boltzmann function (see Materials and Methods, R 2 = 0.81) was fitted to a Rhod‐FF fluorescence trace in response to a voltage ramp (C, C′). iGluSnFR‐AUC was calculated per depolarization voltage and used for a Boltzmann fit (D′, R 2 = 0.92). The voltage of half‐maximal activation (V1/2) and the dynamic range (10–90%) of synaptic Ca2+‐influx and glutamate release were calculated from the fits and depicted as circle and bar in (C′ and D′), respectively.
- E
The obtained fits from the Ca2+ “hot spot” (C′) and from glutamate release (D′) were plotted against each other in a voltage range from −57 to −17 mV in 1 mV increments. A power function was fitted up to the 25% of the maximum iGluSnFR‐AUC (R 2 = 0.99) to obtain the m estimate. (ruptured patch‐clamp, 10 mM intracellular EGTA, 5 mM [Ca2+]e; See also Fig EV4).

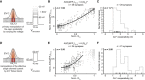
Normalized whole‐cell QCa, calculated in response to 50‐ms‐long step depolarizations, is plotted as a function of depolarization voltage. A Boltzmann function was fitted to estimate the V1/2. Individual IHCs are color coded in shades of blue (mean ± SD, N = 34 IHCs from 28 mice, ruptured patch‐clamp, 10 mM intracellular EGTA, 5 mM [Ca2+]e).
A voltage ramp was applied to obtain ΔF of Rhod‐FF as a proxy of synaptic Ca2+ influx. The V1/2 of synaptic Ca2+ influx was calculated from a modified Boltzmann function (see Materials and Methods) fitted to ΔF/F0. (mean ± SD, n = 55 synapses; individual synapses are color coded; see Appendix Fig S7 for individual fits).
Normalized iGluSnFR‐AUC, in response to 50‐ms‐long step depolarizations, same as (A) (see Appendix Fig S8 for individual fits).
The relation of whole‐cell Ca2+‐influx (A) and the synaptic glutamate release (C).
The relation of synaptic Ca2+ influx (B) and glutamate release (C). The bold lines show the means. (See also Appendix Fig S9 for individual plots)
The histogram shows the Ca2+ cooperativities (m) obtained by individual power function fitting until 25% of normalized iGluSnFR response from ((D); gray) and from ((E); green). The mean m was found to be 1.42 and 2.47, respectively. The rug plot shows the individual data points.
The V1/2 of synaptic Ca2+ influx and glutamate release is correlated (Pearson's r = 0.43, P = 0.0008, Student's t‐test). The marginal histograms show the distribution of each axis. Linear regression analysis (solid lines) and the associated 95% confidence intervals (shaded area). (See also Figs EV3, EV4, EV5).

V1/2 distribution of QCa (gray, N = 34 IHCs), synaptic Ca2+ influx (magenta), and glutamate release (green, n = 55 synapses from 34 IHCs).
Dynamic ranges (10–90%) of the synaptic Ca2+ influx (magenta) and glutamate release (green) with their V1/2 depicted. The synapses are ranked based on their glutamate release threshold (V10). Note how they gradually span the voltage range from −62.48 to −38.41 mV (mean ± SD = 48.27 ± 6.47 mV).

- A
The single cell example shows release dynamics of two synapses innervating the same IHC from either pillar or modiolar side. iGluSnFR signals (band‐stop filtered at 33.3 Hz) in response to 50‐ms‐long step depolarizations to the given depolarization voltages are depicted (ruptured patch‐clamp, 10 mM intracellular EGTA, 5 mM [Ca2+]e). The middle panel shows ΔF images of iGluSnFR, recorded from the mid‐section of the IHC. * depict the first detected response in the given synapse. Note that pillar synapse 1 is already active at −49 mV, while modiolar synapse 2 starts responding only at −41 mV. (Scale bar: 2 μm).
- A′
The normalized iGluSnFR‐AUC as a function of depolarization voltage. Dynamic range and V1/2 of the synapses are depicted in the lower panel.
- B–E
Left panel shows the linear regression analysis (solid lines) of V1/2 of synaptic Ca2+ influx (B), V1/2 (C), threshold (D), and dynamic range (E) of glutamate release as a function of position along the pillar–modiolar axis. Shaded areas depict the associated 95% confidence intervals. Significance of correlation coefficients is reported by a two‐tailed P‐value. Right panel shows box and whisker plots of these properties of synapses grouped into pillar and modiolar halves of the IHCs. Box plots indicate first quartile (25th percentile), median and third quartile (75th percentile) with whiskers reaching from 10 to 90%. For comparison of pillar and modiolar synapses, either Student's t‐test (for normally distributed data) or Mann–Whitney U‐test (for non‐normally distributed data) was applied. *P ≤ 0.05, **P ≤ 0.01.
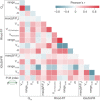

- A–C
The 2D plots of the first three principal components (PCs), labeled based on the clusters obtained by K‐means clustering.
- D
3D plot showing the first three PCs.
- E
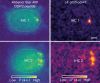
Single IHC exhibits different modes of Ca2+‐control of release. The overlaid mean ΔF image of Rhod‐FF (magenta) and iGluSnFR (green) shows synaptic Ca2+ influx and glutamate release at three neighboring modiolar synapses (individual synapses are color coded based on their clusters).
- E′
The relation between Ca2+ influx and glutamate release of given synapses in the voltage range of −57 to −17 mV plotted with 1 mV increments. A power function was fitted until the 25% of normalized iGluSnFR‐AUC. Synapses showed different Ca2+ dependencies (m = 2.2, 1.4, 7.8; green, purple, orange).
- E″
Dynamic ranges of corresponding Ca2+ influx (gray) and glutamate release (color coded) with V1/2 depicted. Note that the synapses from Cluster 1 (purple), employing Ca2+ nanodomain‐like control of release (m = 1.4), exhibit wider dynamic range than the other synapses.
- F–I
Mean glutamate release (iGluSnFR‐AUC) as a function of depolarization voltage in the three identified clusters (mean ± SEM, Cluster 1: n = 27 synapses, Cluster 2: n = 22 synapses, Cluster 3: n = 6 synapses). The Ca2+ cooperativity (m) (G), dynamic range (H), and glutamate release threshold (V10) (I) show differences for the three clusters. Cluster 1 is composed of linear synapses with wider dynamic range and lower threshold compared to the other clusters. Pillar synapses are depicted as white‐filled circles, and modiolar ones as gray‐filled circles. Box plots indicate first quartile (25th percentile), median and third quartile (75th percentile) with whiskers reaching from 10–90%. The clusters were compared by one‐way ANOVA test, followed by a post hoc Tukey's test. *P ≤ 0.05, **P ≤ 0.01.
- J
The proposed model for sound intensity encoding in an IHC. Differences in the presynaptic control of release, in terms of Ca2+ signaling and Ca2+ channel–exocytosis coupling, enable IHC to diversify the transfer functions of individual synapses for the same receptor potentials.
Comment in
-
Divide and conquer acoustic diversity.EMBO J. 2021 Mar 1;40(5):e107531. doi: 10.15252/embj.2020107531. Epub 2021 Feb 8. EMBO J. 2021. PMID: 33555064 Free PMC article.
References
-
- Ashmore J (2008) Cochlear outer hair cell motility. Physiol Rev 88: 173–210 - PubMed
-
- Beutner D, Voets T, Neher E, Moser T (2001) Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29: 681–690 - PubMed
-
- Böhme MA, Grasskamp AT, Walter AM (2018) Regulation of synaptic release‐site Ca2+ channel coupling as a mechanism to control release probability and short‐term plasticity. FEBS Lett 592: 3516–3531 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

