Bergenin protects pancreatic beta cells against cytokine-induced apoptosis in INS-1E cells
- PMID: 33347462
- PMCID: PMC7751853
- DOI: 10.1371/journal.pone.0241349
Bergenin protects pancreatic beta cells against cytokine-induced apoptosis in INS-1E cells
Abstract
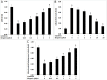
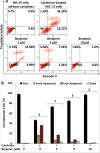
Beta cell apoptosis induced by proinflammatory cytokines is one of the hallmarks of diabetes. Small molecules which can inhibit the cytokine-induced apoptosis could lead to new drug candidates that can be used in combination with existing therapeutic interventions against diabetes. The current study evaluated several effects of bergenin, an isocoumarin derivative, in beta cells in the presence of cytokines. These included (i) increase in beta cell viability (by measuring cellular ATP levels) (ii) suppression of beta cell apoptosis (by measuring caspase activity), (iii) improvement in beta cell function (by measuring glucose-stimulated insulin secretion), and (iv) improvement of beta cells mitochondrial physiological functions. The experiments were carried out using rat beta INS-1E cell line in the presence or absence of bergenin and a cocktail of proinflammatory cytokines (interleukin-1beta, tumor necrosis factor-alpha, and interferon- gamma) for 48 hr. Bergenin significantly inhibited beta cell apoptosis, as inferred from the reduction in the caspase-3 activity (IC50 = 7.29 ± 2.45 μM), and concurrently increased cellular ATP Levels (EC50 = 1.97 ± 0.47 μM). Bergenin also significantly enhanced insulin secretion (EC50 = 6.73 ± 2.15 μM) in INS-1E cells, presumably because of the decreased nitric oxide production (IC50 = 6.82 ± 2.83 μM). Bergenin restored mitochondrial membrane potential (EC50 = 2.27 ± 0.83 μM), decreased ROS production (IC50 = 14.63 ± 3.18 μM), and improved mitochondrial dehydrogenase activity (EC50 = 1.39 ± 0.62 μM). This study shows for the first time that bergenin protected beta cells from cytokine-induced apoptosis and restored insulin secretory function by virtue of its anti-inflammatory, antioxidant and anti-apoptotic properties. To sum up, the above mentioned data highlight bergenin as a promising anti-apoptotic agent in the context of diabetes.
Conflict of interest statement
The authors have read the journal's policy and have the following competing interests: Searle Pharmaceuticals Pakistan Ltd. provided funds for publishing this work. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures



Similar articles
-
Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway.Eur J Pharmacol. 2011 Nov 16;670(1):311-6. doi: 10.1016/j.ejphar.2011.08.033. Epub 2011 Sep 8. Eur J Pharmacol. 2011. PMID: 21925162
-
Polyphenolic extracts from Olea europea L. protect against cytokine-induced β-cell damage through maintenance of redox homeostasis.Rejuvenation Res. 2011 Jun;14(3):325-34. doi: 10.1089/rej.2010.1111. Epub 2011 Jul 11. Rejuvenation Res. 2011. PMID: 21745095
-
Lisofylline, a novel antiinflammatory agent, protects pancreatic beta-cells from proinflammatory cytokine damage by promoting mitochondrial metabolism.Endocrinology. 2002 Jun;143(6):2341-8. doi: 10.1210/endo.143.6.8841. Endocrinology. 2002. PMID: 12021199
-
Identification of Small Molecule Inhibitors that Suppress Cytokine-Induced Apoptosis in Human Pancreatic Islet Cells.2010 Oct 29 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Oct 29 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 22049578 Free Books & Documents. Review.
-
Small-molecule inhibition of inflammatory β-cell death.Diabetes Obes Metab. 2013 Sep;15 Suppl 3(0 3):176-84. doi: 10.1111/dom.12158. Diabetes Obes Metab. 2013. PMID: 24003935 Free PMC article. Review.
Cited by
-
The traditional uses, phytochemistry, pharmacology and toxicology of Bergenia purparescens: A review comments and suggestions.Heliyon. 2023 Nov 16;9(11):e22249. doi: 10.1016/j.heliyon.2023.e22249. eCollection 2023 Nov. Heliyon. 2023. PMID: 38058656 Free PMC article. Review.
-
Molecular Docking and Absorption, Distribution, Metabolism, and Excretion (ADME) Analysis: Examining the Binding Modes and Affinities of Myricetin With Insulin Receptor, Glycogen Synthase Kinase, and Glucokinase.Cureus. 2024 Feb 7;16(2):e53810. doi: 10.7759/cureus.53810. eCollection 2024 Feb. Cureus. 2024. PMID: 38465169 Free PMC article.
-
Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule.Biomolecules. 2023 Feb 21;13(3):403. doi: 10.3390/biom13030403. Biomolecules. 2023. PMID: 36979338 Free PMC article. Review.
-
Bergenin inhibits palmitic acid-induced pancreatic β-cell inflammatory death via regulating NLRP3 inflammasome activation.Ann Transl Med. 2022 Oct;10(19):1058. doi: 10.21037/atm-22-3781. Ann Transl Med. 2022. PMID: 36330410 Free PMC article.
References
-
- Ahmed E, Arshad M, Ahmad M, Saeed M, Ishaque M. Ethnopharmacological survey of some medicinally important plants of Galliyat Areas of NWFP, Pakistan. Asian Journal of Plant Sciences. 2004;3(4):410–5. https://scialert.net/abstract/?doi=ajps.2004.410.415
-
- Da Silva SL, Oliveira VG, Yano T, Nunomura RD. Antimicrobial activity of bergenin from Endopleura uchi (Huber) Cuatrec [Atividade antimicrobiana de bergenina isolada de Endopleura uchi (Huber) Cuatrec]. v. 39, n. 1 2009. 10.1590/S0044-59672009000100019 - DOI
-
- Uddin G, Sadat A, Siddiqui BS. Comparative antioxidant and antiplasmodial activities of 11-O-galloylbergenin and bergenin isolated from Bergenia ligulata. Trop Biomed. 2014. March 1;31(1):143–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

