Decreased diarrheal and respiratory disease in HIV exposed uninfected children following vaccination with rotavirus and pneumococcal conjugate vaccines
- PMID: 33347474
- PMCID: PMC7751865
- DOI: 10.1371/journal.pone.0244100
Decreased diarrheal and respiratory disease in HIV exposed uninfected children following vaccination with rotavirus and pneumococcal conjugate vaccines
Abstract
Background: Rotavirus vaccine (RV) and pneumococcal vaccine (PCV) decrease diarrheal and respiratory disease incidence and severity, but there are few data about the effects of these vaccines among HIV-exposed uninfected (HEU) children.
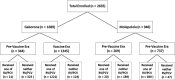
Methods: We recorded RV and PCV vaccination history in a placebo-controlled trial that studied the need for cotrimoxazole among HEU infants in Botswana (the Mpepu Study). We categorized infants by enrollment before or after the simultaneous April 2012 introduction of RV and PCV, and compared diagnoses of diarrhea and pneumonia (grade 3/4), hospitalizations, and deaths from both disease conditions through the 12-month study visit by vaccine era/status across two sites (a city and a village) by Kaplan-Meier estimates.
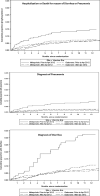
Results: Two thousand six hundred and thirty-five HEU infants were included in this secondary analysis, of these 1689 (64%) were enrolled in Gaborone (344 pre-vaccine, 1345 vaccine) and 946 (36%) in Molepolole (209 pre-vaccine, 737 vaccine). We observed substantial reduction in hazard of hospitalization or death for reason of diarrhea and pneumonia in the vaccine era versus the pre-vaccine era in Molepolole (hazard ratio, HR = 0.44, 95% confidence interval, CI = 0.28, 0.71) with smaller reduction in Gaborone (HR = 0.91, 95% CI = 0.57, 1.45). Similar downward trends were observed for diagnoses of diarrhea and pneumonia separately during the vaccine versus pre-vaccine era.
Conclusions: Although temporal confounding cannot be excluded, significant declines in the burden of diarrheal and respiratory illness were observed among HEU children in Botswana following the introduction of RV and PCV. RV and PCV may maximally benefit HEU children in rural areas with higher disease burden.
Conflict of interest statement
Authors K.B and J.L of Bennett Statistical Consulting and Goodtables Data Consulting respectively were contracted for data curation and management purposes. This does not alter our adherence to PLOS ONE policies on sharing data and materials. All other authors declare that they have no competing interests.
Figures
References
-
- United Nations Inter-Agency Group for Child Mortality Estimation (UN IGME), ‘Levels & Trends in Child Mortality: Report 2018, Estimates developed by the United Nations Inter-Agency Group for Child Mortality Estimation’, United Nations Children’s Fund, New York, 2018. Available from: https://www.unicef.org/publications/index_103264.html
-
- Operario D.J, Platts-Mills J.A, Nadan S, Page N, Seheri M, Mphahlele J et al. (2017). Etiology of Severe Acute Watery Diarrhea in Children in the Global Rotavirus Surveillance Network Using Quantitative Polymerase Chain Reaction. The Journal of Infectious Diseases 2017; 216:220–7. 10.1093/infdis/jix294 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous