Abnormal skeletal muscle blood flow, contractile mechanics and fibre morphology in a rat model of obese-HFpEF
- PMID: 33347612
- PMCID: PMC7898698
- DOI: 10.1113/JP280899
Abnormal skeletal muscle blood flow, contractile mechanics and fibre morphology in a rat model of obese-HFpEF
Abstract
Key points: Heart failure is characterised by limb and respiratory muscle impairments that limit functional capacity and quality of life. However, compared with heart failure with reduced ejection fraction (HFrEF), skeletal muscle alterations induced by heart failure with preserved ejection fraction (HFpEF) remain poorly explored. Here we report that obese-HFpEF induces multiple skeletal muscle alterations in the rat hindlimb, including impaired muscle mechanics related to shortening velocity, fibre atrophy, capillary loss, and an impaired blood flow response to contractions that implies a perfusive oxygen delivery limitation. We also demonstrate that obese-HFpEF is characterised by diaphragmatic alterations similar to those caused by denervation - atrophy in Type IIb/IIx (fast/glycolytic) fibres and hypertrophy in Type I (slow/oxidative) fibres. These findings extend current knowledge in HFpEF skeletal muscle physiology, potentially underlying exercise intolerance, which may facilitate future therapeutic approaches.
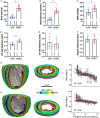
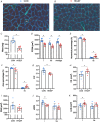
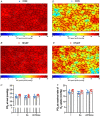
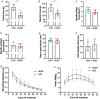
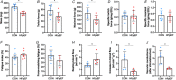
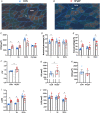
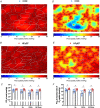
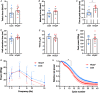
Abstract: Peripheral skeletal muscle and vascular alterations induced by heart failure with preserved ejection fraction (HFpEF) remain poorly identified, with limited therapeutic targets. This study used a cardiometabolic obese-HFpEF rat model to comprehensively phenotype skeletal muscle mechanics, blood flow, microvasculature and fibre atrophy. Lean (n = 8) and obese-HFpEF (n = 8) ZSF1 rats were compared. Skeletal muscles (soleus and diaphragm) were assessed for in vitro contractility (isometric and isotonic properties) alongside indices of fibre-type cross-sectional area, myosin isoform, and capillarity, and estimated muscle PO2 . In situ extensor digitorum longus (EDL) contractility and femoral blood flow were assessed. HFpEF soleus demonstrated lower absolute maximal force by 22%, fibre atrophy by 24%, a fibre-type shift from I to IIa, and a 17% lower capillary-to-fibre ratio despite increased capillary density (all P < 0.05) with preserved muscle PO2 (P = 0.115) and isometric specific force (P > 0.05). Soleus isotonic properties (shortening velocity and power) were impaired by up to 17 and 22%, respectively (P < 0.05), while the magnitude of the exercise hyperaemia was attenuated by 73% (P = 0.012) in line with higher muscle fatigue by 26% (P = 0.079). Diaphragm alterations (P < 0.05) included Type IIx fibre atrophy despite Type I/IIa fibre hypertrophy, with increased indices of capillarity alongside preserved contractile properties during isometric, isotonic, and cyclical contractions. In conclusion, obese-HFpEF rats demonstrated blunted skeletal muscle blood flow during contractions in parallel to microvascular structural remodelling, fibre atrophy, and isotonic contractile dysfunction in the locomotor muscles. In contrast, diaphragm phenotype remained well preserved. This study identifies numerous muscle-specific impairments that could exacerbate exercise intolerance in obese-HFpEF.
Keywords: HFpEF; blood flow; diaphragm; heart failure; muscle atrophy; muscle contraction; skeletal muscle.
© 2020 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
Comment in
-
Unpreserved muscle function in heart failure with preserved ejection fraction: Diffusion limitations defused.J Physiol. 2021 Mar;599(5):1361-1362. doi: 10.1113/JP281204. Epub 2021 Jan 14. J Physiol. 2021. PMID: 33404101 No abstract available.
References
-
- Adams V, Linke A & Winzer E (2017). Skeletal muscle alterations in HFrEF vs. HFpEF. Curr Heart Fail Rep 14, 489–497. - PubMed
-
- Al‐Shammari AA, Kissane RWP, Holbek S, Mackey AL, Andersen TR, Gaffney EA, Kjaer M & Egginton S (2019). Integrated method for quantitative morphometry and oxygen transport modeling in striated muscle. J Appl Physiol 126, 544–557. - PubMed
-
- Aravamudan B, Mantilla CB, Zhan WZ & Sieck GC (2006). Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol 100, 1617–1622. - PubMed
-
- Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD & von Haehling S (2016). Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 222, 41–46. - PubMed
-
- Benson AP, Bernus O, Dierckx H, Gilbert SH, Greenwood JP, Holden AV, Mohee K, Plein S, Radjenovic A, Ries ME, Smith GL, Sourbron S & Walton RD (2011). Construction and validation of anisotropic and orthotropic ventricular geometries for quantitative predictive cardiac electrophysiology. Interface Focus 1, 101–116. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

