Cost-effectiveness of point-of-care testing with task-shifting for HIV care in South Africa: a modelling study
- PMID: 33347810
- PMCID: PMC8284441
- DOI: 10.1016/S2352-3018(20)30279-4
Cost-effectiveness of point-of-care testing with task-shifting for HIV care in South Africa: a modelling study
Abstract
Background: The number of people on antiretroviral therapy (ART) requiring treatment monitoring in low-resource settings is rapidly increasing. Point-of-care (POC) testing for ART monitoring might alleviate burden on centralised laboratories and improve clinical outcomes, but its cost-effectiveness is unknown.
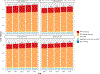
Methods: We used cost and effectiveness data from the STREAM trial in South Africa (February, 2017-October, 2018), which evaluated POC testing for viral load, CD4 count, and creatinine, with task shifting from professional to lower-cadre registered nurses compared with laboratory-based testing without task shifting (standard of care). We parameterised an agent-based network model, EMOD-HIV, to project the impact of implementing this intervention in South Africa over 20 years, simulating approximately 175 000 individuals per run. We assumed POC monitoring increased viral suppression by 9 percentage points, enrolment into community-based ART delivery by 25 percentage points, and switching to second-line ART by 1 percentage point compared with standard of care, as reported in the STREAM trial. We evaluated POC implementation in varying clinic sizes (10-50 patient initiating ART per month). We calculated incremental cost-effectiveness ratios (ICERs) and report the mean and 90% model variability of 250 runs, using a cost-effectiveness threshold of US$500 per disability-adjusted life-year (DALY) averted for our main analysis.
Findings: POC testing at 70% coverage of patients on ART was projected to reduce HIV infections by 4·5% (90% model variability 1·6 to 7·6) and HIV-related deaths by 3·9% (2·0 to 6·0). In clinics with 30 ART initiations per month, the intervention had an ICER of $197 (90% model variability -27 to 863) per DALY averted; results remained cost-effective when varying background viral suppression, ART dropout, intervention effectiveness, and reduction in HIV transmissibility. At higher clinic volumes (≥40 ART initiations per month), POC testing was cost-saving and at lower clinic volumes (20 ART initiations per month) the ICER was $734 (93 to 2569). A scenario that assumed POC testing did not increase enrolment into community ART delivery produced ICERs that exceeded the cost-effectiveness threshold for all clinic volumes.
Interpretation: POC testing is a promising strategy to cost-effectively improve patient outcomes in moderately sized clinics in South Africa. Results are most sensitive to changes in intervention impact on enrolment into community-based ART delivery.
Funding: National Institutes of Health.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
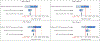
References
-
- UNAIDS: Fast-track, Ending the AIDS epidemic by 2030. Accessed on 1/16/2020 from: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014rep....
-
- WHO Consolidated ARV Guidelines. Accessed from https://www.who.int/hiv/pub/guidelines/arv2013/treatment/en/ on 3/4/2019.
-
- Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with Scale-Up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries, January 2015-June 2016. MMWR Morbidity and mortality weekly report. 2016;65(47):1332–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

