Association of liver abnormalities with in-hospital mortality in patients with COVID-19
- PMID: 33347952
- PMCID: PMC7749734
- DOI: 10.1016/j.jhep.2020.12.012
Association of liver abnormalities with in-hospital mortality in patients with COVID-19
Abstract
Background & aims: The evolution and clinical significance of abnormal liver chemistries and the impact of hepatitis B infection on outcome in patients with COVID-19 is not well characterized. This study aimed to explore these issues.
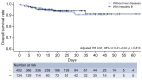
Methods: This large retrospective cohort study included 2,073 patients with coronavirus disease 2019 (COVID-19) and definite outcomes in Wuhan, China. Longitudinal liver function tests were conducted, with associated factors and risk of death determined by multivariate regression analyses. A prognostic nomogram was formulated to predict the survival of patients with COVID-19. The characteristics of liver abnormalities and outcomes of patients with COVID-19, with and without hepatitis B, were compared after 1:3 propensity score matching.
Results: Of the 2,073 patients, 1,282 (61.8%) had abnormal liver chemistries during hospitalization, and 297 (14.3%) had a liver injury. The mean levels of aspartate aminotransferase (AST) and direct bilirubin (D-Bil) increased early after symptom onset in deceased patients and showed disparity compared to levels in discharged patients throughout the clinical course of the disease. Abnormal AST (adjusted hazard ratio [HR] 1.39; 95% CI 1.04-1.86, p = 0.027) and D-Bil (adjusted HR 1.66; 95% CI 1.22-2.26; p = 0.001) levels at admission were independent risk factors for mortality due to COVID-19. A nomogram was established based on the results of multivariate analysis and showed sufficient discriminatory power and good consistency between the prediction and the observation. HBV infection in patients did not increase the risk of poor COVID-19-associated outcomes.
Conclusions: Abnormal AST and D-Bil levels at admission were independent predictors of COVID-19-related mortality. Therefore, monitoring liver chemistries, especially AST and D-Bil levels, is necessary in hospitalized patients with COVID-19.
Lay summary: Liver test abnormalities (in particular elevations in the levels of aspartate aminotransferase [AST] and direct bilirubin [D-Bil]) were observed after symptom onset in patients who went on to die of coronavirus disease 2019 (COVID-19). Abnormal levels of AST and D-Bil at admission were independent predictors of COVID-19-related mortality. HBV infection in patients did not increase the risk of poor COVID-19-associated outcomes.
Keywords: Aspartate aminotransferase; COVID-19; Direct bilirubin; Hepatitis B; Liver injury.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interests All authors declare no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




Comment in
-
Liver injury in COVID-19 - The culprit may not be COVID-19!J Hepatol. 2021 Sep;75(3):739-740. doi: 10.1016/j.jhep.2021.03.010. Epub 2021 Mar 19. J Hepatol. 2021. PMID: 33753154 Free PMC article. No abstract available.
-
Risk stratification in hospitalized COVID-19 patients.J Hepatol. 2021 Sep;75(3):740-742. doi: 10.1016/j.jhep.2021.04.024. Epub 2021 Apr 24. J Hepatol. 2021. PMID: 33901573 Free PMC article. No abstract available.
-
Letter regarding "Association of liver abnormalities with in-hospital mortality in patients with COVID-19".J Hepatol. 2021 Sep;75(3):737-739. doi: 10.1016/j.jhep.2021.04.023. Epub 2021 Apr 24. J Hepatol. 2021. PMID: 33905795 Free PMC article. No abstract available.
-
Reply to: Comments on "Association of liver abnormalities with in-hospital mortality in patients with COVID-19".J Hepatol. 2021 Sep;75(3):742-744. doi: 10.1016/j.jhep.2021.05.027. Epub 2021 Jun 6. J Hepatol. 2021. PMID: 34090927 Free PMC article. No abstract available.
References
-
- National Health Commission of China . 2020. Guidance for COVID-19: Prevention, Control, Diagnosis and Management.http://wwwgovcn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d4... Version 7.0.
-
- World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection issuspected: interim guidance. January 28, 2020. https://wwwwhoint/publications-detail/clinical-management-of-severeacute...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

