Results of Testing Children for Severe Acute Respiratory Syndrome Coronavirus-2 Through a Community-based Testing Site
- PMID: 33347958
- PMCID: PMC7831849
- DOI: 10.1016/j.jpeds.2020.12.030
Results of Testing Children for Severe Acute Respiratory Syndrome Coronavirus-2 Through a Community-based Testing Site
Abstract
Objective: To describe the demographics, clinical features, and test results of children referred from their primary provider for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the community setting.
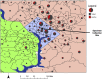
Study design: Retrospective cross-sectional study of children ≤22 years of age who were tested for SARS-CoV-2 at a community-based specimen collection site in Washington, DC, affiliated with a large children's hospital between March 21 and May 16, 2020.
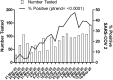
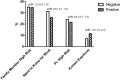
Results: Of the 1445 patients tested at the specimen collection site for SARS-CoV-2 virus, 408 (28.2%) had a positive polymerase chain reaction test. The daily positivity rate increased over the study period, from 5.4% during the first week to a peak of 47.4% (Ptrend < .001). Patients with fever (aOR, 1.7; 95% CI, 1.3-2.3) or cough (aOR, 1.4; 95% CI, 1.1-1.9) and those with known contact with someone with confirmed SARS-CoV-2 infection (aOR, 1.6; 95% CI, 1.0-2.4.) were more likely have a positive test, but these features were not highly discriminating.
Conclusions: In this cohort of mildly symptomatic or well children and adolescents referred to a community drive-through/walk-up SARS-CoV-2 testing site because of risk of exposure or clinical illness, 1 in 4 patients had a positive test. Children and young adults represent a considerable burden of SARS-CoV-2 infection. Assessment of their role in transmission is essential to implementing appropriate control measures.
Keywords: COVID-19; SARS-CoV-2; asymptomatic; children; mildly symptomatic; pediatric.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
References
-
- Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S., et al. Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

