Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment
- PMID: 33348052
- PMCID: PMC8116573
- DOI: 10.1016/j.ymthe.2020.12.024
Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment
Abstract
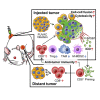
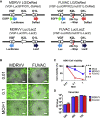
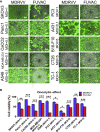
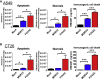
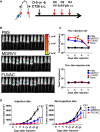
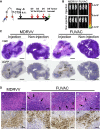
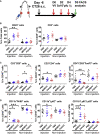
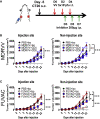
Oncolytic viruses induce antitumor immunity following direct viral oncolysis. However, their therapeutic effects are limited in distant untreated tumors because their antitumor function depends on indirect antitumor immunity. Here, we generated a novel fusogenic oncolytic vaccinia virus (FUVAC) and compared its antitumor activity with that of its parental non-fusogenic virus. Compared with the parent, FUVAC exerted the cytopathic effect and induced immunogenic cell death in human and murine cancer cells more efficiently. In a bilateral tumor-bearing syngeneic mouse model, FUVAC administration significantly inhibited tumor growth in both treated and untreated tumors. However, its antitumor effects were completely suppressed by CD8+ T cell depletion. Notably, FUVAC reduced the number of tumor-associated immune-suppressive cells in treated tumors, but not in untreated tumors. Mice treated with FUVAC before an immune checkpoint inhibitor (ICI) treatment achieved complete response (CR) in both treated and untreated tumors, whereas ICI alone did not show antitumor activity. Mice achieving CR rejected rechallenge with the same tumor cells, suggesting establishment of a long-term tumor-specific immune memory. Thus, FUVAC improves the tumor immune microenvironment and enhances systemic antitumor immunity, suggesting that, alone and in combination with ICI, it is a novel immune modulator for overcoming oncolytic virus-resistant tumors.
Keywords: antitumor immunity; cell membrane fusion; immune checkpoint blockade; oncolytic function; vaccinia virus.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Andtbacka R.H., Ross M., Puzanov I., Milhem M., Collichio F., Delman K.A., Amatruda T., Zager J.S., Cranmer L., Hsueh E. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM Phase III clinical trial. Ann. Surg. Oncol. 2016;23:4169–4177. - PMC - PubMed
-
- Kaufman H.L., Amatruda T., Reid T., Gonzalez R., Glaspy J., Whitman E., Harrington K., Nemunaitis J., Zloza A., Wolf M., Senzer N.N. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J. Immunother. Cancer. 2016;4:12. - PMC - PubMed
-
- Park B.H., Hwang T., Liu T.C., Sze D.Y., Kim J.S., Kwon H.C., Oh S.Y., Han S.Y., Yoon J.H., Hong S.H. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

