Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations
- PMID: 33348372
- PMCID: PMC8008989
- DOI: 10.1667/RADE-20-00065.1
Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations
Abstract
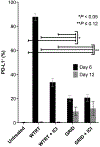
The combination of radiotherapy and immunotherapy may generate synergistic anti-tumor host immune responses and promote abscopal effects. Spatial fractionation of a radiation dose has been found to promote unique physiological responses of tumors, which might promote synergy with immunotherapy. To determine whether spatial fractionation may augment immune activity, whole-tumor or spatial fractionation grid radiation treatment (GRID) alone or in combination with antibodies against immune checkpoints PD1 and CTLA-4 were tested in an immunocompetent mouse model using a triple negative breast tumor (4T1). Tumor growth delay, immunohistochemistry and flow cytometry were used to characterize the effects of each treatment type. Whole-beam radiation with immune checkpoint inhibition significantly restrained tumor growth in the irradiated tumor, but not abscopal tumors, compared to either of these treatments alone. In mice that received spatially fractionated irradiation, evidence of abscopal immune responses were observed in contralateral tumors with markedly enhanced infiltration of both antigen-presenting cells and activated T cells, which were preceded by increased systemic IFNγ production and led to eventual tumor growth delay. These studies suggest that systemic immune activation may be triggered by employing GRID to a primary tumor lesion, promoting anti-tumor immune responses outside the treatment field. Interestingly, PD-L1 was found to be upregulated in abscopal tumors from GRID-treated mice. Combined radio-immunotherapy therapy is becoming a validated and novel approach in the treatment of cancer. With the potential increased benefit of GRID to augment both local and metastatic disease responses, further exploration of GRID treatment as a part of current standards of care is warranted.
©2020 by Radiation Research Society. All rights of reproduction in any form reserved.
Figures






References
-
- Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019; 393:2051–8. - PubMed
-
- Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019; 5:1276–82. - PMC - PubMed
-
- Milano MT, Zhang H, Metcalfe SK, Muhs AG, Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat 2009; 115:601–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

