Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: A multicentre, randomised, double-blind, placebo-controlled Phase II trial
- PMID: 33348420
- PMCID: PMC8048941
- DOI: 10.1002/ijc.33453
Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: A multicentre, randomised, double-blind, placebo-controlled Phase II trial
Abstract
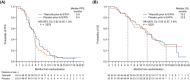
Trilaciclib is an intravenous CDK4/6 inhibitor administered prior to chemotherapy to preserve haematopoietic stem and progenitor cells and immune system function from chemotherapy-induced damage (myelopreservation). The effects of administering trilaciclib prior to carboplatin, etoposide and atezolizumab (E/P/A) were evaluated in a randomised, double-blind, placebo-controlled Phase II study in patients with newly diagnosed extensive-stage small cell lung cancer (ES-SCLC) (NCT03041311). The primary endpoints were duration of severe neutropenia (SN; defined as absolute neutrophil count <0.5 × 109 cells per L) in Cycle 1 and occurrence of SN during the treatment period. Other endpoints were prespecified to assess the effects of trilaciclib on additional measures of myelopreservation, patient-reported outcomes, antitumour efficacy and safety. Fifty-two patients received trilaciclib prior to E/P/A and 53 patients received placebo. Compared to placebo, administration of trilaciclib resulted in statistically significant decreases in the mean duration of SN in Cycle 1 (0 vs 4 days; P < .0001) and occurrence of SN (1.9% vs 49.1%; P < .0001), with additional improvements in red blood cell and platelet measures and health-related quality of life (HRQoL). Trilaciclib was well tolerated, with fewer grade ≥3 adverse events compared with placebo, primarily due to less high-grade haematological toxicity. Antitumour efficacy outcomes were comparable. Administration of trilaciclib vs placebo generated more newly expanded peripheral T-cell clones (P = .019), with significantly greater expansion among patients with an antitumour response to E/P/A (P = .002). Compared with placebo, trilaciclib administered prior to E/P/A improved patients' experience of receiving treatment for ES-SCLC, as shown by reduced myelosuppression, and improved HRQoL and safety profiles.
Keywords: chemotherapy; myelopreservation; myelosuppression; small cell lung cancer (SCLC); trilaciclib.
© 2020 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Dr Davey Daniel has received research funding from G1 Therapeutics, Inc and Genentech. Dr Jana Jaal has received research funding from AstraZeneca, and has been an adviser to AstraZeneca, Boehringer Ingelheim and MSD. Dr Lowell Hart has received research funding, consultancy and travel expenses from G1 Therapeutics, Inc. Dr Yili Pritchett and Dr Jessica A. Sorrentino are paid employees and shareowners of G1 Therapeutics, Inc. Dr Shannon R. Morris and Joyce M. Antal were employees of G1 Therapeutics, Inc at the time of manuscript preparation and submission. Dr Vladimer Kuchava, Prof. Igor Bondarenko, Dr Oleksandr Ivashchuk, Dr Sreekanth Reddy, Dr Iveta Kudaba and Dr Amiran Matitashvili have no conflicts of interest to declare. Dr Jerome Goldschmidt has participated in speakers bureau and received honoraria from Amgen and Bristol Myers Squibb.
Figures



References
-
- Loehrer PJ Sr, Einhorn LH, Greco FA. Cisplatin plus etoposide in small cell lung cancer. Semin Oncol. 1988;15:2‐8. - PubMed
-
- Eckardt JR, von Pawel J, Papai Z, et al. Open‐label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy‐naive patients with extensive‐disease small‐cell lung cancer. J Clin Oncol. 2006;24:2044‐2051. - PubMed
-
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med. 2002;346:85‐91. - PubMed
-
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive‐stage disease small‐cell lung cancer. J Clin Oncol. 2006;24:2038‐2043. - PubMed
-
- Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy‐naive patients with extensive‐stage small‐cell lung cancer. J Clin Oncol. 2009;27:4787‐4792. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

