Non-pharmacological care for opioid withdrawal in newborns
- PMID: 33348423
- PMCID: PMC8130993
- DOI: 10.1002/14651858.CD013217.pub2
Non-pharmacological care for opioid withdrawal in newborns
Abstract
Background: The prevalence of substance use, both prescribed and non-prescribed, is increasing in many areas of the world. Substance use by women of childbearing age contributes to increasing rates of neonatal abstinence syndrome (NAS). Neonatal opioid withdrawal syndrome (NOWS) is a newer term describing the subset of NAS related to opioid exposure. Non-pharmacological care is the first-line treatment for substance withdrawal in newborns. Despite the widespread use of non-pharmacological care to mitigate symptoms of NAS, there is not an established definition of, and standard for, non-pharmacological care practices in this population. Evaluation of safety and efficacy of non-pharmacological practices could provide clear guidance for clinical practice.
Objectives: To evaluate the safety and efficacy of non-pharmacological treatment of infants at risk for, or having symptoms consistent with, opioid withdrawal on the length of hospitalization and use of pharmacological treatment for symptom management. Comparison 1: in infants at risk for, or having early symptoms consistent with, opioid withdrawal, does non-pharmacological treatment reduce the length of hospitalization and use of pharmacological treatment? Comparison 2: in infants receiving pharmacological treatment for symptoms consistent with opioid withdrawal, does concurrent non-pharmacological treatment reduce duration of pharmacological treatment, maximum and cumulative doses of opioid medication, and length of hospitalization?
Search methods: We used the standard search strategy of Cochrane Neonatal to search CENTRAL (2019, Issue 10); Ovid MEDLINE; and CINAHL on 11 October 2019. We also searched clinical trials databases and the reference lists of retrieved articles for randomized controlled trials (RCTs), quasi-RCTs, and cluster trials.
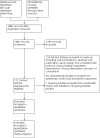
Selection criteria: We included trials comparing single or bundled non-pharmacological interventions to no non-pharmacological treatment or different single or bundled non-pharmacological interventions. We assessed non-pharmacological interventions independently and in combination based on sufficient similarity in population, intervention, and comparison groups studied. We categorized non-pharmacological interventions as: modifying environmental stimulation, feeding practices, and support of the mother-infant dyad. We presented non-randomized studies identified in the search process narratively.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We used the GRADE approach to assess the certainty of evidence. Primary outcomes in infants at risk for, or having early symptoms consistent with, opioid withdrawal included length of hospitalization and pharmacological treatment with one or more doses of opioid or sedative medication. Primary outcomes in infants receiving opioid treatment for symptoms consistent with opioid withdrawal included length of hospitalization, length of pharmacological treatment with opioid or sedative medication, and maximum and cumulative doses of opioid medication.
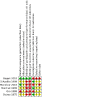
Main results: We identified six RCTs (353 infants) in which infants at risk for, or having symptoms consistent with, opioid withdrawal participated between 1975 and 2018. We identified no RCTs in which infants receiving opioid treatment for symptoms consistent with opioid withdrawal participated. The certainty of evidence for all outcomes was very low to low. We also identified and excluded 34 non-randomized studies published between 2005 and 2018, including 29 in which infants at risk for, or having symptoms consistent with, opioid withdrawal participated and five in which infants receiving opioid treatment for symptoms consistent with opioid withdrawal participated. We identified seven preregistered interventional clinical trials that may qualify for inclusion at review update when complete. Of the six RCTs, four studies assessed modifying environmental stimulation in the form of a mechanical rocking bed, prone positioning, non-oscillating waterbed, or a low-stimulation nursery; one study assessed feeding practices (comparing 24 kcal/oz to 20 kcal/oz formula); and one study assessed support of the maternal-infant dyad (tailored breastfeeding support). There was no evidence of a difference in length of hospitalization in the one study that assessed modifying environmental stimulation (mean difference [MD) -1 day, 95% confidence interval [CI) -2.82 to 0.82; 30 infants; very low-certainty evidence) and the one study of support of the maternal-infant dyad (MD -8.9 days, 95% CI -19.84 to 2.04; 14 infants; very low-certainty evidence). No studies of feeding practices evaluated the length of hospitalization. There was no evidence of a difference in use of pharmacological treatment in three studies of modifying environmental stimulation (typical risk ratio [RR) 1.00, 95% CI 0.86 to 1.16; 92 infants; low-certainty evidence), one study of feeding practices (RR 0.92, 95% CI 0.63 to 1.33; 49 infants; very low-certainty evidence), and one study of support of the maternal-infant dyad (RR 0.50, 95% CI 0.13 to 1.90; 14 infants; very low-certainty evidence). Reported secondary outcomes included neonatal intensive care unit (NICU) admission, days to regain birth weight, and weight nadir. One study of support of the maternal-infant dyad reported NICU admission (RR 0.50, 95% CI 0.13 to 1.90; 14 infants; very low-certainty evidence). One study of feeding practices reported days to regain birth weight (MD 1.10 days, 95% CI 2.76 to 0.56; 46 infants; very low-certainty evidence). One study that assessed modifying environmental stimulation reported weight nadir (MD -0.28, 95% CI -1.15 to 0.59; 194 infants; very low-certainty evidence) and one study of feeding practices reported weight nadir (MD -0.8, 95% CI -2.24 to 0.64; 46 infants; very low-certainty evidence).
Authors' conclusions: We are uncertain whether non-pharmacological care for opioid withdrawal in newborns affects important clinical outcomes including length of hospitalization and use of pharmacological treatment based on the six included studies. The outcomes identified for this review were of very low- to low-certainty evidence. Combined analysis was limited by heterogeneity in study design and intervention definitions as well as the number of studies. Many prespecified outcomes were not reported. Although caregivers are encouraged by experts to optimize non-pharmacological care for opioid withdrawal in newborns prior to initiating pharmacological care, we do not have sufficient evidence to inform specific clinical practices. Larger well-designed studies are needed to determine the effect of non-pharmacological care for opioid withdrawal in newborns.
Trial registration: ClinicalTrials.gov NCT02768844 NCT02801331 NCT03097484 NCT03533985 NCT03549936 NCT03987165.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AP: none.
LY: none.
MEBF: none.
LM has worked with Vermont Oxford Network since 2012 and received payments for lectures.
RS is the Co‐ordinating Editor of Cochrane Neonatal, President and Director of Clinical Trials of the Vermont Oxford Network, and a professor at the University of Vermont. Previously he has acted as a consultant and invited speaker for several of the pharmaceutical companies that manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs Wellcome). He has done no paid consulting work since 2010.
Figures






Update of
- doi: 10.1002/14651858.CD013217
References
References to studies included in this review
Bogen 2018 {published data only}
D'Apolito 1999 {published data only}
MacVicar 2018 {published data only}
Maichuk 1999 {published data only}
Oro 1988 {published data only}
Ostrea 1975 {published data only}
-
- Ostrea EM, Chavez CJ, Strauss ME. A study of factors that influence the severity of neonatal narcotic withdrawal. Addictive Diseases 1975;2(1-2):187-99. [PMID: ] - PubMed
References to studies excluded from this review
Abdel‐Latif 2006 {published data only}
-
- Abdel-Latif ME, Pinner J, Clews S, Cooke F, Lui K, Oei J. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics 2006;117(6):e1163-9. [DOI: ] [PMID: ] - PubMed
Abrahams 2007 {published data only}
Abrahams 2010 {published data only}
-
- Abrahams RR, Mackay-Dunn MH, Nevmerjitskaia V, Macrae GS, Payne SP, Hodgson ZG. An evaluation of rooming-in among substance-exposed newborns in British Columbia. Journal d'Obstetrique et Gynecologie du Canada : JOGC [Journal of Obstetrics and Gynaecology Canada : JOGC] 2010;32(9):866-71. [DOI: ] [PMID: ] - PubMed
Arlettaz 2005 {published data only}
-
- Arlettaz R, Kashiwagi M, Das-Kundu S, Fauchère JC, Lang A, Bucher HU. Methadone maintenance program in pregnancy in a Swiss perinatal center (II): neonatal outcome and social resources. Acta Obstetricia et Gynecologica Scandinavica 2005;84(2):145-50. [DOI: ] [PMID: ] - PubMed
Crook 2017 {published data only}
Dryden 2009 {published data only}
-
- Dryden C, Young D, Campbell N, Mactier H. Postnatal weight loss in substitute methadone-exposed infants: implications for the management of breast feeding. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(3):F214-6. [DOI: ] [PMID: ] - PubMed
Grossman 2017 {published data only}
Hodgson 2012 {published data only}
-
- Hodgson ZG, Abrahams RR. A rooming-in program to mitigate the need to treat for opiate withdrawal in the newborn. Journal d'Obstetrique et Gynecologie du Canada : JOGC [Journal of Obstetrics and Gynaecology Canada : JOGC] 2012;34(5):475-81. [DOI: 10.1016/s1701-2163(16)35245-8] [PMID: ] - DOI - PubMed
Holmes 2016 {published data only}
-
- Holmes AV, Atwood EC, Whalen B, Beliveau J, Jarvis JD, Matulis JC, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics 2016;137(6):e1-e9. [DOI: ] [PMID: ] - PubMed
Howard 2017 {published data only}
Hünseler 2013 {published data only}
-
- Hünseler C, Brückle M, Roth B, Kribs A. Neonatal opiate withdrawal and rooming-in: a retrospective analysis of a single center experience [Neonataler opiatentzug und rooming-in: retrospektive analyse der erfahrungen eines zentrums]. Klinische Pädiatrie 2013;225(5):247-51. [DOI: ] [PMID: ] - PubMed
Isemann 2011 {published data only}
-
- Isemann B, Meinzen-Derr J, Akinbi H. Maternal and neonatal factors impacting response to methadone therapy in infants treated for neonatal abstinence syndrome. Journal of Perinatology 2011;31(1):25-9. [DOI: ] [PMID: ] - PubMed
Jansson 2008 {published data only}
-
- Jansson LM, Choo R, Velez ML, Harrow C, Schroeder JR, Shakleya DM, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics 2008;121(1):106-14. [DOI: ] [PMID: ] - PubMed
Kirchner 2014 {published data only}
-
- Kirchner L, Graf-Rohrmeister K, Klebermass-Schrehof K, Weninger M, Jagsch R, Metz V, et al. Neonatal abstinence syndrome in European and North American neonates: differences in clinical characteristics derived from a prospective randomized trial [Neonatales Abstinenzsyndrom bei europäischen und nordamerikani-schen Neugeborenen: Unterschiede im klinischen Verlauf an Hand vonDaten einer prospektiven randomisierten Studie]. Klinische Padiatrie 2014;226(5):274-80. [DOI: 10.1055/s-0034-1372586] [PMID: ] - DOI - PubMed
Liu 2015 {published data only}
Loudin 2017 {published data only}
McKnight 2016 {published data only}
-
- McKnight S, Coo H, Davies G, Holmes B, Newman A, Newton L, et al. Rooming-in for infants at risk of neonatal abstinence syndrome. American Journal of Perinatology 2016;33(5):495-501. [DOI: ] [PMID: ] - PubMed
McQueen 2011 {published data only}
-
- McQueen KA, Murphy-Oikonen J, Gerlach K, Montelpare W. The impact of infant feeding method on neonatal abstinence scores of methadone-exposed infants. Advances in Neonatal Care 2011;11(4):282-90. [DOI: ] [PMID: ] - PubMed
Metz 2011 {published data only}
Metz 2015 {published data only}
-
- Metz VE, Comer SD, Pribasnig A, Wuerzl J, Fischer G. Observational study in an outpatient clinic specializing in treating opioid dependent pregnant women: neonatal abstinence syndrome in infants exposed to methadone-, buprenorphine- and slow-release oral morphine. Heroin Addiction and Related Clinical Problems 2015;17(1):5-16.
Miles 2007 {published data only}
-
- Miles J, Sugumar K, Macrory F, Sims DG, D'Souza SW. Methadone-exposed newborn infants: outcome after alterations to a service for mothers and infants. Child Care Health and Development 2007;33(2):206-12. [DOI: ] [PMID: ] - PubMed
O'Connor 2013 {published data only}
-
- O'Connor AB, Collett A, Alto WA, O'Brien LM. Breastfeeding rates and the relationship between breastfeeding and neonatal abstinence syndrome in women maintained on buprenorphine during pregnancy. Journal of Midwifery and Women's Health 2013;58(4):383-8. [DOI: ] [PMID: ] - PubMed
Ordean 2015 {published data only}
-
- Ordean A, Kahan M, Graves L, Abrahams R, Kim T. Obstetrical and neonatal outcomes of methadone-maintained pregnant women: a Canadian multisite cohort study. Journal d'Obstetrique et Gynecologie du Canada : JOGC [Journal of Obstetrics and Gynaecology Canada : JOGC] 2015;37(3):252-7. [DOI: ] [PMID: ] - PubMed
Patrick 2016 {published data only}
Pritham 2012 {published data only}
Radmacher 2017 {published data only}
-
- Radmacher P, Alexander C, Devlin L. Donor human milk may decrease severe gastrointestinal distress in infants with neonatal abstinence syndrome. Journal of Pregnancy and Neonatal Medicine 2017;1(1):11-5. [DOI: 10.35841/neonatal-medicine.1.1.11-15] - DOI
Radziewicz 2018 {published data only}
Saiki 2010 {published data only}
-
- Saiki T, Lee S, Hannam S, Greenough A. Neonatal abstinence syndrome-postnatal ward versus neonatal unit management. European Journal of Pediatrics 2010;169(1):95-8. [DOI: ] [PMID: ] - PubMed
Short 2016 {published data only}
-
- Short VL, Gannon M, Abatemarco DJ. The association between breastfeeding and length of hospital stay among infants diagnosed with neonatal abstinence syndrome: a population-based study of in-hospital births. Breastfeeding Medicine 2016;11(7):343-9. [DOI: ] [PMID: ] - PubMed
Summey 2018 {published data only}
-
- Summey J, Chen L, Mayo R, Charron E, Hudson JA, Sherrill WW, et al. Early treatment innovation for opioid-dependent newborns: a retrospective comparison of outcomes, utilization, quality, and safety, 2006–2014. Joint Commission Journal on Quality and Patient Safety / Joint Commission Resources 2018;44(6):312-20. [DOI: ] [PMID: ] - PubMed
Wachman 2013 {published data only}
Wachman 2018 {published data only}
-
- Wachman EM, Grossman M, Schiff DM, Philipp B, Minear S, Hutton E, et al. Quality improvement initiative to improve inpatient outcomes for neonatal abstinence syndrome. Journal of Perinatology 2018;38(8):1114-22. [DOI: ] [PMID: ] - PubMed
Welle‐Strand 2013 {published data only}
-
- Welle-Strand GK, Skurtveit S, Jansson LM, Bakstad B, Bjarko L, Ravndal E. Breastfeeding reduces the need for withdrawal treatment in opioid-exposed infants. Acta Paediatrica 2013;102(11):1060-6. [DOI: ] [PMID: 23909865] - PubMed
References to ongoing studies
NCT02768844 {published data only}
-
- NCT02768844. Physiology and therapeutic management of neonatal abstinence syndrome. clinicaltrials.gov/show/NCT02768844 (first received 11 May 2016).
NCT02801331 {published data only}
-
- NCT02801331. Efficacy and outcomes of a non-pharmacological intervention for neonatal abstinence syndrome. clinicaltrials.gov/show/NCT02801331 (first received 15 June 2016).
NCT03097484 {published data only}
-
- NCT03097484. The effect of aromatherapy on neonatal abstinence syndrome and salivary cortisol levels. clinicaltrials.gov/show/NCT03097484 (first received 31 March 2017).
NCT03113656 {published data only}
-
- NCT03113656. Weighted blankets with infants with NAS. clinicaltrials.gov/show/NCT03113656 (first received 13 April 2017).
NCT03533985 {published data only}
-
- NCT03533985. Effect of music therapy on Infants with Neonatal Abstinence Syndrome. clinicaltrials.gov/show/NCT03533985 (first received 23 May 2018).
NCT03549936 {published data only}
-
- NCT03549936. Role of low lactose infant formula in the management of neonatal abstinence syndrome. clinicaltrials.gov/show/NCT03549936 (first received 8 June 2018).
NCT03987165 {published data only}
-
- NCT03987165. The effect of music therapy on newborns. clinicaltrials.gov/show/NCT03987165 (first received 14 June 2019).
Additional references
Arditi 2006
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development-II. San Antonio (TX): Psychological Corporation, 1993.
Bembich 2018
Bogen 2017
Bogen 2019
Brockington 2006
Brogly 2014
Burns 2007
Carbajal 2003
Chaudhary 2013
-
- Chaudhary T, Walch E, Herold B, Metze B, Lejeune A, Burkhardt F, et al. Predictive and concurrent validity of standardized neurodevelopmental examinations by the Griffiths scales and Bayley Scales of Infant Development II. Klinische Padiatrie 2013;225(1):8-12. [DOI: 10.1055/s-0032-1331169] [PMID: ] - DOI - PubMed
Chen 2017
CIHI 2018
-
- Canadian Institute for Health Information. Opioid-related harms in Canada, 2018. cihi.ca/sites/default/files/document/opioid-related-harms-report-2018-en... (assessed prior to 1 December 2020).
Condon 1998
-
- Condon J, Corkindale CJ. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. Journal of Reproductive and Infant Psychology 1998;16(1):57-76. [DOI: 10.1080/02646839808404558] - DOI
Corr 2017
Cox 1987
Davies 2016
-
- Davies H, Gilbert R, Johnson K, Petersen I, Nazareth I, O'Donnell M, et al. Neonatal drug withdrawal syndrome: cross-country comparison using hospital administrative data in England, the USA, Western Australia, and Ontario, Canada. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(1):F26-30. [DOI: 10.1136/archdischild-2015-308948] [PMID: ] - DOI - PubMed
Devlin 2020
Faherty 2019
Fenton 2017
Finnegan 1975
-
- Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive Diseases 1975;2(1-2):141-58. [PMID: ] - PubMed
Gomez Pomar 2017
Goyal 2020
-
- Goyal S, Saunders KC, Moore CS, Fillo K, Ko J, Manning S, et al. Identification of substance-exposed newborns and neonatal abstinence syndrome using ICD-10-CM – 15 Hospitals, Massachusetts, 2017. Morbidity and Mortality Weekly Report 2020;69(29):951-5. [DOI: 10.15585/mmwr.mm6929a2] [PMID: ] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 2 February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Graham 2010
Gray 2000
Gray 2002
Green 1981
Grossman 2018
Hall 2015
HCUP 2019
-
- Healthcare Cost and Utilization Project. HCUP Fast Stats, 2019. hcup-us.ahrq.gov/news/announcements/faststats092019update.jsp (accessed prior to 1 December 2020).
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PMC - PubMed
Hudak 2012
Jansson 2009
Johnston 2017
Jones 2010
Jones 2016a
Jones 2016b
Juneau 2015
Kaplan 2008
Kelly 2020
-
- Kelly L, Shan F, MacVicar S, Czaplinkski E, Moulsdale W, Simpson S, et al. A core outcome set for neonatal opioid withdrawal syndrome. Pediatrics 2020;146(1):e20200018. [DOI: ] [PMID: ] - PubMed
Ko 2016
Kocherlakota 2020
-
- Kocherlakota P, Qian E, Patel VC, Mandru C, Vilar RE, Alpan G, et al. A new scoring system for the assessment of neonatal abstinence syndrome. American Journal of Perinatology 2020;37(3):333-40. [DOI: ] [PMID: ] - PubMed
Krans 2019
Kroenke 2001
Larson 2019
Lee 2020
-
- Lee S, Bora S, Austin N, Westerman A, Hernderson J. Neurodevelopmental outcomes of children born to opioid-dependent mothers: a systematic review and meta-analysis. Academic Pediatrics 2020;20(3):308-18. [DOI: ] [PMID: ] - PubMed
Lemon 2018
Li 2009
Liaw 2012
-
- Liaw J, Yang L, Wang K, Chen C, Chang Y, Yin T. Non-nutritive sucking and facilitated tucking relieve preterm infant pain during heel-stick procedures: a prospective, randomised controlled crossover trial. International Journal of Nursing Studies 2012;49(3):300-9. [DOI: 10.1016/j.ijnurstu.2011.09.017] [PMID: ] - DOI - PubMed
Lipsitz 1975
Lisonkova 2019
-
- Lisonkova S, Richter L, Ting J, Muraca G, Mehrabadi A, Mitchell-Foster S, et al. Neonatal abstinence syndrome and associated neonatal and maternal mortality and morbidity. Pediatrics 2019;144(2):e20183664. [DOI: ] [PMID: ] - PubMed
MacMillan 2018
MacVicar 2019
Maguire 2013
Mangat 2019
McQueen 2019
Mehta 2013
Milliren 2018
-
- Milliren CE, Gupta M, Graham DA, Melvin P, Jorina M, Ozonoff A. Hospital variation in neonatal abstinence syndrome incidence, treatment modalities, resource use, and costs across pediatric hospitals in the United States, 2013 to 2016. Hospital Pediatrics 2018;8(1):15-20. [DOI: 10.1542/hpeds.2017-0077] [PMID: ] - DOI - PubMed
Moe‐Byrne 2018
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: ] [PMID: ] - PubMed
Moon 2016
Muller 1994
-
- Muller ME. A questionnaire to measure mother-to-infant attachment. Journal of Nursing Measurement 1994;2(2):129-41. [PMID: ] - PubMed
Newman 2015
O'Donnell 2009
-
- O'Donnell M, Nassar N, Leonard H, Hagan R, Mathews R, Patterson Y, et al. Increasing prevalence of neonatal withdrawal syndrome: population study of maternal factors and child protection involvement. Pediatrics 2009;123(4):e614-21. [DOI: ] [PMID: ] - PubMed
O'Grady 2009
Oostlander 2019
Osborn 2010a
Osborn 2010b
Palisano 1997
Paltrow 2013
Pandita 2018
Patrick 2012
Patrick 2015a
Patrick 2015b
Patrick 2019
Pinelli 2002
Raith 2015
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, Cochrane, 2020.
Ryan 2019
Sarkar 2006
Schmidt 2007
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shah 2012
Shan 2020
Smith 2018
Snowden 2019
Sterne 2016
Stine 2009
-
- Stine SM, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Fischer G, et al. Characteristics of opioid-using pregnant women who accept or refuse participation in a clinical trial: screening results from the MOTHER study. American Journal of Drug and Alcohol Abuse 2009;35(6):429-33. [DOI: 10.3109/00952990903374080] - DOI - PMC - PubMed
Taylor 2005
Tolia 2015
Uebel 2015
-
- Uebel H, Wright IM, Burns L, Hilder L, Bajuk B, Breen C, et al. Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatrics 2015;136(4):e811-20. [DOI: ] [PMID: ] - PubMed
Uebel 2016
Velez 2009
Wachman 2018a
-
- Wachman EM, Warden AH, Thomas Z, Thomas-Lewis JA, Shrestha H, Nikita FN, et al. Impact of psychiatric medication co-exposure on neonatal abstinence syndrome severity. Drug and Alcohol Dependence 2018;192:45-50. [DOI: ] [PMID: ] - PubMed
Wachman 2018b
Whalen 2019
Whooley 1997
Winkelman 2018
Yeoh 2019
Zahorodny 1998
-
- Zahorodny W, Rom C, Whitney W, Giddens S, Samuel M, Maichuk G, et al. The neonatal withdrawal inventory: a simplified score of newborn withdrawal. Journal of Developmental and Behavioral Pediatrics 1998;19(2):89-93. [DOI: ] [PMID: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

