Vasa Vasorum Lumen Narrowing in Brain Vascular Hyalinosis in Systemic Hypertension Patients Who Died of Ischemic Stroke
- PMID: 33348552
- PMCID: PMC7767198
- DOI: 10.3390/ijms21249611
Vasa Vasorum Lumen Narrowing in Brain Vascular Hyalinosis in Systemic Hypertension Patients Who Died of Ischemic Stroke
Abstract
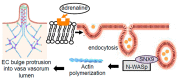
Ischemic stroke is a major cause of death among patients with systemic hypertension. The narrowing of the lumen of the brain vasculature contributes to the increased incidence of stroke. While hyalinosis represents the major pathological lesions contributing to vascular lumen narrowing and stroke, the pathogenic mechanism of brain vascular hyalinosis has not been well characterized. Thus, the present study examined the postmortem brain vasculature of human patients who died of ischemic stroke due to systemic hypertension. Hematoxylin and eosin staining and immunohistochemistry showed the occurrence of brain vascular hyalinosis with infiltrated plasma proteins along with the narrowing of the vasa vasorum and oxidative stress. Transmission electron microscopy revealed endothelial cell bulge protrusion into the vasa vasorum lumen and the occurrence of endocytosis in the vasa vasorum endothelium. The treatment of cultured microvascular endothelial cells with adrenaline also promoted the formation of the bulge as well as endocytic vesicles. The siRNA knockdown of sortin nexin-9 (a mediator of clathrin-mediated endocytosis) inhibited adrenaline-induced endothelial cell bulge formation. Adrenaline promoted protein-protein interactions between sortin nexin-9 and neural Wiskott-Aldrich syndrome protein (a regulator of actin polymerization). Spontaneously hypertensive stroke-prone rats also exhibited lesions indicative of brain vascular hyalinosis, the endothelial cell protrusion into the lumen of the vasa vasorum, and endocytosis in vasa vasorum endothelial cells. We propose that endocytosis-dependent endothelial cell bulge protrusion narrows the vasa vasorum, resulting in ischemic oxidative damage to cerebral vessels, the formation of hyalinosis, the occurrence of ischemic stroke, and death in systemic hypertension patients.
Keywords: brain; hyalinosis; ischemic stroke; oxidative stress; vasa vasorum; vascular.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart disease and stroke Statistics-2019 update: A report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
-
- Heuschmann P.U., Wiedmann S., Wellwood I., Rudd A., Di Carlo A., Bejot Y., Ryglewicz D., Rastenyte D., Wolfe C.D., European Registers of Stroke Three-month stroke outcome: The European Registers of Stroke (EROS) investigators. Neurology. 2011;76:159–165. doi: 10.1212/WNL.0b013e318206ca1e. - DOI - PubMed
-
- Filipets O.O., Pashkovsky V.M. Stroke burden in Ukraine: Analysis of the official stroke statistics and overview of population-based epidemiological studies. Clin. Exp. Pathol. 2014;13:189–193.
-
- Kulesh S.D., Filina N.A., Frantava N.M., Zhytko N.L., Kastsinevich T.M., Kliatskova L.A., Shumskas M.S., Hilz M.J., Schwab S., Kolominsky-Rabas P.L. Incidence and case-fatality of stroke on the East border of the European union: The Grodno Stroke Study. Stroke. 2010;41:2726–2730. doi: 10.1161/STROKEAHA.110.596916. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

