Quantification of Trastuzumab-HER2 Engagement In Vitro and In Vivo
- PMID: 33348564
- PMCID: PMC7767145
- DOI: 10.3390/molecules25245976
Quantification of Trastuzumab-HER2 Engagement In Vitro and In Vivo
Abstract
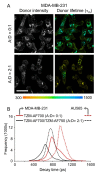
Human EGF Receptor 2 (HER2) is an important oncogene driving aggressive metastatic growth in up to 20% of breast cancer tumors. At the same time, it presents a target for passive immunotherapy such as trastuzumab (TZM). Although TZM has been widely used clinically since 1998, not all eligible patients benefit from this therapy due to primary and acquired drug resistance as well as potentially lack of drug exposure. Hence, it is critical to directly quantify TZM-HER2 binding dynamics, also known as cellular target engagement, in undisturbed tumor environments in live, intact tumor xenograft models. Herein, we report the direct measurement of TZM-HER2 binding in HER2-positive human breast cancer cells and tumor xenografts using fluorescence lifetime Forster Resonance Energy Transfer (FLI-FRET) via near-infrared (NIR) microscopy (FLIM-FRET) as well as macroscopy (MFLI-FRET) approaches. By sensing the reduction of fluorescence lifetime of donor-labeled TZM in the presence of acceptor-labeled TZM, we successfully quantified the fraction of HER2-bound and internalized TZM immunoconjugate both in cell culture and tumor xenografts in live animals. Ex vivo immunohistological analysis of tumors confirmed the binding and internalization of TZM-HER2 complex in breast cancer cells. Thus, FLI-FRET imaging presents a powerful analytical tool to monitor and quantify cellular target engagement and subsequent intracellular drug delivery in live HER2-positive tumor xenografts.
Keywords: FRET imaging; HER2; fluorescence lifetime; immunoconjugate; target engagement; trastuzumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Fluorescence Lifetime Imaging for Quantification of Targeted Drug Delivery in Varying Tumor Microenvironments.bioRxiv [Preprint]. 2024 Mar 17:2024.01.12.575453. doi: 10.1101/2024.01.12.575453. bioRxiv. 2024. Update in: Adv Sci (Weinh). 2025 Jan;12(3):e2403253. doi: 10.1002/advs.202403253. PMID: 38293105 Free PMC article. Updated. Preprint.
-
Fluorescence Lifetime Imaging for Quantification of Targeted Drug Delivery in Varying Tumor Microenvironments.Adv Sci (Weinh). 2025 Jan;12(3):e2403253. doi: 10.1002/advs.202403253. Epub 2024 Nov 27. Adv Sci (Weinh). 2025. PMID: 39600235 Free PMC article.
-
HER2-Targeted PET Imaging and Therapy of Hyaluronan-Masked HER2-Overexpressing Breast Cancer.Mol Pharm. 2020 Jan 6;17(1):327-337. doi: 10.1021/acs.molpharmaceut.9b01091. Epub 2019 Dec 19. Mol Pharm. 2020. PMID: 31804840 Free PMC article.
-
Recent Insights into the Development of Preclinical Trastuzumab- Resistant HER2+ Breast Cancer Models.Curr Med Chem. 2018;25(17):1976-1998. doi: 10.2174/0929867323666161216144659. Curr Med Chem. 2018. PMID: 27993109 Review.
-
PF-05280014: A Trastuzumab Biosimilar.BioDrugs. 2018 Oct;32(5):515-518. doi: 10.1007/s40259-018-0308-z. BioDrugs. 2018. PMID: 30280367 Review.
Cited by
-
In vitro and in vivo NIR fluorescence lifetime imaging with a time-gated SPAD camera.Optica. 2022 May;9(5):532-544. doi: 10.1364/OPTICA.454790. Epub 2022 May 9. Optica. 2022. PMID: 35968259 Free PMC article.
-
Luminescence lifetime imaging of three-dimensional biological objects.J Cell Sci. 2021 May 1;134(9):1-17. doi: 10.1242/jcs.254763. Epub 2021 May 7. J Cell Sci. 2021. PMID: 33961054 Free PMC article.
-
Fluorescence Lifetime Imaging for Quantification of Targeted Drug Delivery in Varying Tumor Microenvironments.bioRxiv [Preprint]. 2024 Mar 17:2024.01.12.575453. doi: 10.1101/2024.01.12.575453. bioRxiv. 2024. Update in: Adv Sci (Weinh). 2025 Jan;12(3):e2403253. doi: 10.1002/advs.202403253. PMID: 38293105 Free PMC article. Updated. Preprint.
-
Modeling Pharmacokinetics and Pharmacodynamics of Therapeutic Antibodies: Progress, Challenges, and Future Directions.Pharmaceutics. 2021 Mar 21;13(3):422. doi: 10.3390/pharmaceutics13030422. Pharmaceutics. 2021. PMID: 33800976 Free PMC article. Review.
-
Fluorescence Lifetime Imaging for Quantification of Targeted Drug Delivery in Varying Tumor Microenvironments.Adv Sci (Weinh). 2025 Jan;12(3):e2403253. doi: 10.1002/advs.202403253. Epub 2024 Nov 27. Adv Sci (Weinh). 2025. PMID: 39600235 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

