Next Generation Sequencing-Based Profiling of Cell Free DNA in Patients with Advanced Non-Small Cell Lung Cancer: Advantages and Pitfalls
- PMID: 33348595
- PMCID: PMC7766403
- DOI: 10.3390/cancers12123804
Next Generation Sequencing-Based Profiling of Cell Free DNA in Patients with Advanced Non-Small Cell Lung Cancer: Advantages and Pitfalls
Abstract
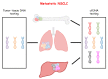
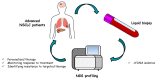
Lung cancer (LC) is the main cause of death for cancer worldwide and non-small cell lung cancer (NSCLC) represents the most common histology. The discovery of genomic alterations in driver genes that offer the possibility of therapeutic intervention has completely changed the approach to the diagnosis and therapy of advanced NSCLC patients, and tumor molecular profiling has become mandatory for the choice of the most appropriate therapeutic strategy. However, in approximately 30% of NSCLC patients tumor tissue is inadequate for biomarker analysis. The development of highly sensitive next generation sequencing (NGS) technologies for the analysis of circulating cell-free DNA (cfDNA) is emerging as a valuable alternative to assess tumor molecular landscape in case of tissue unavailability. Additionally, cfDNA NGS testing can better recapitulate NSCLC heterogeneity as compared with tissue testing. In this review we describe the main advantages and limits of using NGS-based cfDNA analysis to guide the therapeutic decision-making process in advanced NSCLC patients, to monitor the response to therapy and to identify mechanisms of resistance early. Therefore, we provide evidence that the implementation of cfDNA NGS testing in clinical research and in the clinical practice can significantly improve precision medicine approaches in patients with advanced NSCLC.
Keywords: NSCLC; cfDNA; liquid biopsy; next generation sequencing; targeted therapy.
Conflict of interest statement
N.N.: Personal financial interests (speaker’s fees and/or advisory boards): MSD, QIAGEN, Bayer, Biocartis, Incyte, Roche, BMS, MERCK, Thermo Fisher, Boehringer Ingelheim, AstraZeneca, Sanofi, Eli Lilly, Illumina, and Amgen Institutional; financial interests (financial support to research projects): MERCK, Sysmex, Thermo Fisher, QIAGEN, Roche, AstraZeneca, Biocartis, and Illumina. A.M.: MSD, BMS, Boehringer, Pfizer, Roche, AstraZeneca; Advisory Board: Takeda. The other authors declare no conflicts of interests.
Figures
References
-
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. - DOI - PubMed
-
- Sequist L.V., Yang J.C., Yamamoto N., O’Byrne K., Hirsh V., Mok T., Geater S.L., Orlov S., Tsai C.M., Boyer M., et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources