Suppression of Pax3-MITF-M Axis Protects from UVB-Induced Skin Pigmentation by Tetrahydroquinoline Carboxamide
- PMID: 33348800
- PMCID: PMC7766340
- DOI: 10.3390/ijms21249631
Suppression of Pax3-MITF-M Axis Protects from UVB-Induced Skin Pigmentation by Tetrahydroquinoline Carboxamide
Abstract
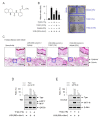
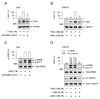
Paired box gene 3 (Pax3) and cAMP responsive element-binding protein (CREB) directly interact with the cis-acting elements on the promoter of microphthalmia-associated transcription factor isoform M (MITF-M) for transcriptional activation in the melanogenic process. Tyrosinase (Tyro) is a target gene of MITF-M, and functions as a key enzyme in melanin biosynthesis. Tetrahydroquinoline carboxamide (THQC) was previously screened as an antimelanogenic candidate. In the current study, we evaluated the antimelanogenic activity of THQC in vivo and elucidated a possible mechanism. Topical treatment with THQC mitigated ultraviolet B (UVB)-induced skin pigmentation in guinea pig with decreased messenger RNA (mRNA) and protein levels of melanogenic genes such as MITF-M and Tyro. Moreover, THQC inhibited cAMP-induced melanin production in α-melanocyte-stimulating hormone (α-MSH)- or histamine-activated B16-F0 cells, in which it suppressed the expression of the MITF-M gene at the promoter level. As a mechanism, THQC normalized the protein levels of Pax3, a transcriptional activator of the MITF-M gene, in UVB-exposed and pigmented skin, as well as in α-MSH-activated B16-F0 culture. However, THQC did not affect UVB- or α-MSH-induced phosphorylation (activation) of CREB. The results suggest that suppression of the Pax3-MITF-M axis might be a potential strategy in the treatment of skin pigmentary disorders that are at high risk under UVB radiation.
Keywords: guinea pig skin; melanin pigmentation; microphthalmia-associated transcription factor isoform M; paired box gene 3; tetrahydroquinoline carboxamide; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Interruption of p38MAPK-MSK1-CREB-MITF-M pathway to prevent hyperpigmentation in the skin.Int J Biol Sci. 2024 Feb 17;20(5):1688-1704. doi: 10.7150/ijbs.93120. eCollection 2024. Int J Biol Sci. 2024. PMID: 38481807 Free PMC article.
-
cAMP-dependent activation of protein kinase A as a therapeutic target of skin hyperpigmentation by diphenylmethylene hydrazinecarbothioamide.Br J Pharmacol. 2015 Jul;172(13):3434-45. doi: 10.1111/bph.13134. Epub 2015 Apr 24. Br J Pharmacol. 2015. PMID: 25766244 Free PMC article.
-
Downregulation of α-Melanocyte-Stimulating Hormone-Induced Activation of the Pax3-MITF-Tyrosinase Axis by Sorghum Ethanolic Extract in B16F10 Melanoma Cells.Int J Mol Sci. 2018 Jun 1;19(6):1640. doi: 10.3390/ijms19061640. Int J Mol Sci. 2018. PMID: 29865165 Free PMC article.
-
Cyclic AMP a key messenger in the regulation of skin pigmentation.Pigment Cell Res. 2000 Apr;13(2):60-9. doi: 10.1034/j.1600-0749.2000.130203.x. Pigment Cell Res. 2000. PMID: 10841026 Review.
-
Skin pigmentation and its control: From ultraviolet radiation to stem cells.Exp Dermatol. 2021 Apr;30(4):560-571. doi: 10.1111/exd.14260. Epub 2020 Dec 24. Exp Dermatol. 2021. PMID: 33320376 Free PMC article. Review.
Cited by
-
Self-assembled gel microneedle formed by MS deep eutectic solvent as a transdermal delivery system for hyperpigmentation treatment.Mater Today Bio. 2024 May 14;26:101090. doi: 10.1016/j.mtbio.2024.101090. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38800564 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

