New Insights into X-Chromosome Reactivation during Reprogramming to Pluripotency
- PMID: 33348832
- PMCID: PMC7766869
- DOI: 10.3390/cells9122706
New Insights into X-Chromosome Reactivation during Reprogramming to Pluripotency
Abstract
Dosage compensation between the sexes results in one X chromosome being inactivated during female mammalian development. Chromosome-wide transcriptional silencing from the inactive X chromosome (Xi) in mammalian cells is erased in a process termed X-chromosome reactivation (XCR), which has emerged as a paradigm for studying the reversal of chromatin silencing. XCR is linked with germline development and induction of naive pluripotency in the epiblast, and also takes place upon reprogramming somatic cells to induced pluripotency. XCR depends on silencing of the long non-coding RNA (lncRNA) X inactive specific transcript (Xist) and is linked with the erasure of chromatin silencing. Over the past years, the advent of transcriptomics and epigenomics has provided new insights into the transcriptional and chromatin dynamics with which XCR takes place. However, multiple questions remain unanswered about how chromatin and transcription related processes enable XCR. Here, we review recent work on establishing the transcriptional and chromatin kinetics of XCR, as well as discuss a model by which transcription factors mediate XCR not only via Xist repression, but also by direct targeting of X-linked genes.
Keywords: TAD; XCI; XCR; Xist; chromatin; embryo development; epigenetics; gene regulation; polycombs; reprogramming.
Conflict of interest statement
The authors declare no known conflict of interest. The funders had no role the writing of the manuscript.
Figures
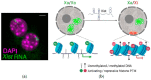
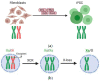
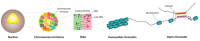
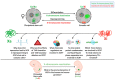
References
-
- Ohno S. Sex Chromosomes and Sex-Linked Genes. Springer; Berlin/Heidelberg, Germany: 1967.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

