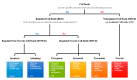
Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies
- PMID: 33348858
- PMCID: PMC7767016
- DOI: 10.3390/cells9122709
Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies
Abstract
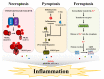
The treatment of tumors requires the induction of cell death. Radiotherapy, chemotherapy, and immunotherapy are administered to kill cancer cells; however, some cancer cells are resistant to these therapies. Therefore, effective treatments require various strategies for the induction of cell death. Regulated cell death (RCD) is systematically controlled by intracellular signaling proteins. Apoptosis and autophagy are types of RCD that are morphologically different from necrosis, while necroptosis, pyroptosis, and ferroptosis are morphologically similar to necrosis. Unlike necrosis, regulated necrotic cell death (RNCD) is caused by disruption of the plasma membrane under the control of specific proteins and induces tissue inflammation. Various types of RNCD, such as necroptosis, pyroptosis, and ferroptosis, have been used as therapeutic strategies against various tumor types. In this review, the mechanisms of necroptosis, pyroptosis, and ferroptosis are described in detail, and a potential effective treatment strategy to increase the anticancer effects on apoptosis- or autophagy-resistant tumor types through the induction of RNCD is suggested.
Keywords: apoptosis; autophagy; ferroptosis; necroptosis; necrosis; pyroptosis; therapy-resistant tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy.Signal Transduct Target Ther. 2022 Jun 20;7(1):196. doi: 10.1038/s41392-022-01046-3. Signal Transduct Target Ther. 2022. PMID: 35725836 Free PMC article. Review.
-
Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation.Cells. 2021 Feb 28;10(3):515. doi: 10.3390/cells10030515. Cells. 2021. PMID: 33671004 Free PMC article. Review.
-
Distinct Types of Regulated Cell Death in Melanoma.Cells. 2025 Jun 1;14(11):823. doi: 10.3390/cells14110823. Cells. 2025. PMID: 40497999 Free PMC article. Review.
-
Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research.J Hematol Oncol. 2022 Dec 8;15(1):174. doi: 10.1186/s13045-022-01392-3. J Hematol Oncol. 2022. PMID: 36482419 Free PMC article. Review.
-
Ferroptosis, necroptosis, and pyroptosis in anticancer immunity.J Hematol Oncol. 2020 Aug 10;13(1):110. doi: 10.1186/s13045-020-00946-7. J Hematol Oncol. 2020. PMID: 32778143 Free PMC article. Review.
Cited by
-
Connexin32 activates necroptosis through Src-mediated inhibition of caspase 8 in hepatocellular carcinoma.Cancer Sci. 2021 Sep;112(9):3507-3519. doi: 10.1111/cas.14994. Epub 2021 Jul 16. Cancer Sci. 2021. PMID: 34050696 Free PMC article.
-
A Pyroptosis-Based Prognostic Model for Immune Microenvironment Estimation of Hepatocellular Carcinoma.Dis Markers. 2022 Jan 10;2022:8109771. doi: 10.1155/2022/8109771. eCollection 2022. Dis Markers. 2022. PMID: 35047095 Free PMC article.
-
In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L.Molecules. 2024 May 23;29(11):2460. doi: 10.3390/molecules29112460. Molecules. 2024. PMID: 38893336 Free PMC article.
-
A Mini Review on Molecules Inducing Caspase-Independent Cell Death: A New Route to Cancer Therapy.Molecules. 2022 Sep 28;27(19):6401. doi: 10.3390/molecules27196401. Molecules. 2022. PMID: 36234938 Free PMC article. Review.
-
Predicting the prognosis of hepatocellular carcinoma based on the interaction between pyroptosis, apoptosis, and necroptosis.Clin Exp Med. 2023 Oct;23(6):2087-2104. doi: 10.1007/s10238-022-00910-4. Epub 2022 Oct 22. Clin Exp Med. 2023. PMID: 36271962
References
-
- Copur M.S. State of Cancer Research Around the Globe. Oncology (Williston Park) 2019;33:181–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

