Preparative Scale Production of Recombinant Human Transthyretin for Biophysical Studies of Protein-Ligand and Protein-Protein Interactions
- PMID: 33348885
- PMCID: PMC7766448
- DOI: 10.3390/ijms21249640
Preparative Scale Production of Recombinant Human Transthyretin for Biophysical Studies of Protein-Ligand and Protein-Protein Interactions
Abstract
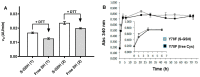
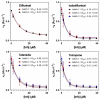
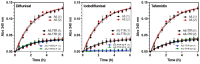
Human transthyretin (hTTR), a serum protein with a main role in transporting thyroid hormones and retinol through binding to the retinol-binding protein, is an amyloidogenic protein involved in familial amyloidotic polyneuropathy (FAP), familial amyloidotic cardiomyopathy, and central nervous system selective amyloidosis. hTTR also has a neuroprotective role in Alzheimer disease, being the major Aβ binding protein in human cerebrospinal fluid (CSF) that prevents amyloid-β (Aβ) aggregation with consequent abrogation of toxicity. Here we report an optimized preparative expression and purification protocol of hTTR (wt and amyloidogenic mutants) for in vitro screening assays of TTR ligands acting as amyloidogenesis inhibitors or acting as molecular chaperones to enhance the TTR:Aβ interaction. Preparative yields were up to 660 mg of homogenous protein per L of culture in fed-batch bioreactor. The recombinant wt protein is mainly unmodified at Cys10, the single cysteine in the protein sequence, whereas the highly amyloidogenic Y78F variant renders mainly the S-glutathionated form, which has essentially the same amyloidogenic behavior than the reduced protein with free Cys10. The TTR production protocol has shown inter-batch reproducibility of expression and protein quality for in vitro screening assays.
Keywords: amyloid diseases; fed-batch culture; protein yield; protein-ligand interactions; protein-protein interactions; recombinant expression; transthyretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Kinetic assay for high-throughput screening of in vitro transthyretin amyloid fibrillogenesis inhibitors.J Comb Chem. 2005 Mar-Apr;7(2):246-52. doi: 10.1021/cc049849s. J Comb Chem. 2005. PMID: 15762752
-
Effect of albumin on transthyretin and amyloidogenic transthyretin Val30Met disposition and tissue deposition in familial amyloidotic polyneuropathy.Life Sci. 2013 Dec 18;93(25-26):1017-22. doi: 10.1016/j.lfs.2013.10.031. Epub 2013 Nov 8. Life Sci. 2013. PMID: 24211615
-
Production of recombinant human transthyretin with biological activities toward the understanding of the molecular basis of familial amyloidotic polyneuropathy (FAP).Biochemistry. 1991 Mar 5;30(9):2415-21. doi: 10.1021/bi00223a017. Biochemistry. 1991. PMID: 1848097
-
Natural compounds as inhibitors of transthyretin amyloidosis and neuroprotective agents: analysis of structural data for future drug design.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1145-1162. doi: 10.1080/14756366.2020.1760262. J Enzyme Inhib Med Chem. 2020. PMID: 32419519 Free PMC article. Review.
-
Methods to evaluate the inhibition of TTR fibrillogenesis induced by small ligands.Curr Med Chem. 2012;19(15):2343-55. doi: 10.2174/092986712800269281. Curr Med Chem. 2012. PMID: 22471983 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

