Extracellular Vesicles: Messengers of p53 in Tumor-Stroma Communication and Cancer Metastasis
- PMID: 33348923
- PMCID: PMC7766631
- DOI: 10.3390/ijms21249648
Extracellular Vesicles: Messengers of p53 in Tumor-Stroma Communication and Cancer Metastasis
Abstract
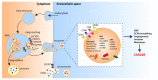
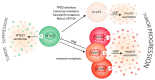
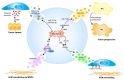
Tumor progression to a metastatic and ultimately lethal stage relies on a tumor-supporting microenvironment that is generated by reciprocal communication between tumor and stromal host cells. The tumor-stroma crosstalk is instructed by the genetic alterations of the tumor cells-the most frequent being mutations in the gene Tumor protein p53 (TP53) that are clinically correlated with metastasis, drug resistance and poor patient survival. The crucial mediators of tumor-stroma communication are tumor-derived extracellular vesicles (EVs), in particular exosomes, which operate both locally within the primary tumor and in distant organs, at pre-metastatic niches as the future sites of metastasis. Here, we review how wild-type and mutant p53 proteins control the secretion, size, and especially the RNA and protein cargo of tumor-derived EVs. We highlight how EVs extend the cell-autonomous tumor suppressive activity of wild-type p53 into the tumor microenvironment (TME), and how mutant p53 proteins switch EVs into oncogenic messengers that reprogram tumor-host communication within the entire organism so as to promote metastatic tumor cell dissemination.
Keywords: exosomes; extracellular vesicles; metastatic niche priming; mutant p53; p53; pre-metastatic niche; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

