Cavitation Emissions Nucleated by Definity Infused through an EkoSonic Catheter in a Flow Phantom
- PMID: 33349516
- PMCID: PMC11537209
- DOI: 10.1016/j.ultrasmedbio.2020.10.010
Cavitation Emissions Nucleated by Definity Infused through an EkoSonic Catheter in a Flow Phantom
Erratum in
-
Corrigendum to 'Cavitation emissions nucleated by Definity infused through an EkoSonic catheter in a flow phantom' [Ultrasound in Med & Biol. 47 (2021) 693-709].Ultrasound Med Biol. 2023 Jan;49(1):410-414. doi: 10.1016/j.ultrasmedbio.2022.09.012. Epub 2022 Oct 18. Ultrasound Med Biol. 2023. PMID: 36266144 Free PMC article. No abstract available.
Abstract
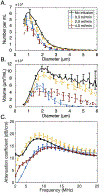
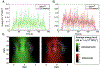
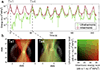
The EkoSonic endovascular system has been cleared by the U.S. Food and Drug Administration for the controlled and selective infusion of physician specified fluids, including thrombolytics, into the peripheral vasculature and the pulmonary arteries. The objective of this study was to explore whether this catheter technology could sustain cavitation nucleated by infused Definity, to support subsequent studies of ultrasound-mediated drug delivery to diseased arteries. The concentration and attenuation spectroscopy of Definity were assayed before and after infusion at 0.3, 2.0 and 4.0 mL/min through the EkoSonic catheter. PCI was used to map and quantify stable and inertial cavitation as a function of Definity concentration in a flow phantom mimicking the porcine femoral artery. The 2.0 mL/min infusion rate yielded the highest surviving Definity concentration and acoustic attenuation. Cavitation was sustained throughout each 15 ms ultrasound pulse, as well as throughout the 3 min infusion. These results demonstrate a potential pathway to use cavitation nucleation to promote drug delivery with the EkoSonic endovascular system.
Keywords: Catheter delivery of Definity; Cavitation nucleation; Drug delivery; Intravascular ultrasound; Theragnostic ultrasound.
Copyright © 2020 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest disclosure Boston Scientific provided the EkoSonic catheters and a driving unit. Curtis Genstler is an employee of Boston Scientific. Alexander S. Hannah is a former employee of Boston Scientific. The other authors have no conflicts of interest to disclose.
Figures


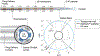


References
-
- Apfel RE. The Role of Impurities in Cavitation-Threshold Determination. J Acoust Soc Am Acoustical Society of America, 1970;48:1179–1186.
-
- Arvanitis CD, Bazan-Peregrino M, Rifai B, Seymour LW, Coussios CC. Cavitation-enhanced extravasation for drug delivery. Ultrasound Med Biol 2011;37:1838–1852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

