Severe respiratory disease caused by human respiratory syncytial virus impairs language learning during early infancy
- PMID: 33349647
- PMCID: PMC7752900
- DOI: 10.1038/s41598-020-79140-1
Severe respiratory disease caused by human respiratory syncytial virus impairs language learning during early infancy
Abstract
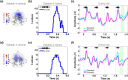
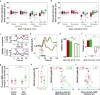
Human respiratory syncytial virus infection is a leading cause of pediatric morbidity and mortality. A previous murine study showed that during severe acute respiratory infections the virus invades the central nervous system, and that infected animals evolve with long-lasting learning difficulties associated with long-term potentiation impairment in their hippocampus. We hypothesized here that human infants who presented a severe episode of respiratory syncytial virus infection before 6 months of age would develop long-term learning difficulties. We measured the acquisition of the native phoneme repertoire during the first year, a milestone in early human development, comprising a reduction in the sensitivity to the irrelevant nonnative phonetic information and an increase in the sensitivity to the information relevant for the native one. We found that infants with a history of severe respiratory infection by the human respiratory syncytial virus presented poor distinction of native and nonnative phonetic contrasts at 6 months of age, and remained atypically sensitive to nonnative contrasts at 12 months, which associated with weak communicative abilities. Our results uncover previously unknown long-term language learning difficulties associated with a single episode of severe respiratory infection by the human respiratory syncytial virus, which could relate to memory impairments.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A 2-year-old girl with chronic crackles after respiratory syncytial virus infection: a case report.J Med Case Rep. 2018 Sep 12;12(1):258. doi: 10.1186/s13256-018-1797-6. J Med Case Rep. 2018. PMID: 30205845 Free PMC article.
-
Long-term consequences of respiratory syncytial virus acute lower respiratory tract infection in early childhood in Guinea-bissau.Pediatr Infect Dis J. 2006 Nov;25(11):1025-31. doi: 10.1097/01.inf.0000243214.80794.3a. Pediatr Infect Dis J. 2006. PMID: 17072125
-
Immune monitoring of children with respiratory syncytial virus infection.Expert Rev Clin Immunol. 2013 May;9(5):393-5. doi: 10.1586/eci.13.20. Expert Rev Clin Immunol. 2013. PMID: 23634732 No abstract available.
-
Respiratory syncytial virus pneumonia: mechanisms of inflammation and prolonged airway hyperresponsiveness.Curr Opin Infect Dis. 2005 Jun;18(3):199-204. doi: 10.1097/01.qco.0000168378.07110.72. Curr Opin Infect Dis. 2005. PMID: 15864095 Review.
-
Testing models predicting severity of respiratory syncytial virus infection on the PICNIC RSV database. Pediatric Investigators Collaborative Network on Infections in Canada.Arch Pediatr Adolesc Med. 1995 Nov;149(11):1217-20. doi: 10.1001/archpedi.1995.02170240035005. Arch Pediatr Adolesc Med. 1995. PMID: 7581752 Review.
Cited by
-
Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders.Pharmaceuticals (Basel). 2021 Apr 8;14(4):340. doi: 10.3390/ph14040340. Pharmaceuticals (Basel). 2021. PMID: 33917837 Free PMC article. Review.
-
Characterization of the humoral and cellular immunity induced by a recombinant BCG vaccine for the respiratory syncytial virus in healthy adults.Front Immunol. 2023 Jul 18;14:1215893. doi: 10.3389/fimmu.2023.1215893. eCollection 2023. Front Immunol. 2023. PMID: 37533867 Free PMC article.
-
Role of G2-S16 Polyanionic Carbosilane Dendrimer in the Prevention of Respiratory Syncytial Virus Infection In Vitro and In Vivo in Mice.Polymers (Basel). 2021 Jun 29;13(13):2141. doi: 10.3390/polym13132141. Polymers (Basel). 2021. PMID: 34209827 Free PMC article.
-
New Developments and Challenges in Antibody-Based Therapies for the Respiratory Syncytial Virus.Infect Drug Resist. 2023 Apr 8;16:2061-2074. doi: 10.2147/IDR.S379660. eCollection 2023. Infect Drug Resist. 2023. PMID: 37063935 Free PMC article. Review.
-
Exploring Therapeutic Targets From Spreading Patterns Against Respiratory Syncytial Virus.FASEB J. 2025 Jul 31;39(14):e70858. doi: 10.1096/fj.202500509RR. FASEB J. 2025. PMID: 40689767 Free PMC article. Review.
References
-
- Flores, J. C., et al. A. Potential neurocognitive consequences of infection by human respiratory syncytial virus. Rev. Chilena Infectol. 33, 537–542. (2016). 10.4067/s0716-10182016000500008 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

