Responsive principles and applications of smart materials in biosensing
- PMID: 33349813
- PMCID: PMC7371594
- DOI: 10.1016/j.smaim.2020.07.001
Responsive principles and applications of smart materials in biosensing
Abstract
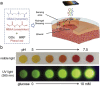
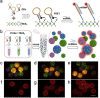
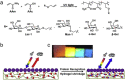
Biosensing is a rising analytical field for detection of biological indicators using transducing systems. Smart materials can response to external stimuli, and translate the stimuli from biological domains into signals that are readable and quantifiable. Smart materials, such as nanomaterials, photonic crystals and hydrogels have been widely used for biosensing purpose. In this review, we illustrate the incorporation of smart materials in biosensing systems, including the design of responsive materials, their responsive mechanism of biosensing, and their applications in detection of four types of common biomolecules (including glucose, nucleic acids, proteins, and enzymes). In the end, we also illustrate the current challenges and prospective of using smart materials in biosensing research fields.
Keywords: Biosensing; Hydrogel; Nanomaterial; Photonic crystal; Responsive material.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









Similar articles
-
Stimuli-Responsive Crystalline Smart Materials: From Rational Design and Fabrication to Applications.Acc Chem Res. 2022 Apr 5;55(7):1047-1058. doi: 10.1021/acs.accounts.2c00027. Epub 2022 Mar 16. Acc Chem Res. 2022. PMID: 35294183
-
Stimuli-responsive DNA-based hydrogels for biosensing applications.J Nanobiotechnology. 2022 Jan 21;20(1):40. doi: 10.1186/s12951-022-01242-x. J Nanobiotechnology. 2022. PMID: 35062945 Free PMC article. Review.
-
From design to applications of stimuli-responsive hydrogel strain sensors.J Mater Chem B. 2020 Apr 29;8(16):3171-3191. doi: 10.1039/c9tb02692d. J Mater Chem B. 2020. PMID: 31998926 Review.
-
DNA Hydrogels and Microgels for Biosensing and Biomedical Applications.Adv Mater. 2020 Jan;32(3):e1806538. doi: 10.1002/adma.201806538. Epub 2019 Aug 5. Adv Mater. 2020. PMID: 31379017 Review.
-
Application of smart-responsive hydrogels in nucleic acid and nucleic acid-based target sensing: A review.Biosens Bioelectron. 2025 Jan 1;267:116803. doi: 10.1016/j.bios.2024.116803. Epub 2024 Sep 19. Biosens Bioelectron. 2025. PMID: 39316868 Review.
Cited by
-
Emerging Bioanalytical Devices and Platforms for Rapid Detection of Pathogens in Environmental Samples.Micromachines (Basel). 2022 Jul 8;13(7):1083. doi: 10.3390/mi13071083. Micromachines (Basel). 2022. PMID: 35888900 Free PMC article. Review.
-
Emerging Theranostic Nanomaterials in Diabetes and Its Complications.Adv Sci (Weinh). 2022 Jan;9(3):e2102466. doi: 10.1002/advs.202102466. Epub 2021 Nov 25. Adv Sci (Weinh). 2022. PMID: 34825525 Free PMC article. Review.
-
Novabeads: Stimuli-Responsive Signal-Amplifying Hydrogel Microparticles for Enzymeless Fluorescence-Based Detection of microRNA Biomarkers.Small. 2025 Aug;21(33):e2503990. doi: 10.1002/smll.202503990. Epub 2025 Jun 25. Small. 2025. PMID: 40557570 Free PMC article.
-
4D-Printed Redox-Responsive Needle-Flow Reactors Enabling Online Quantitative Profiling of Living Rat Brain Extracellular Lactate and Glucose.Anal Chem. 2025 Jul 29;97(29):15642-15650. doi: 10.1021/acs.analchem.5c01036. Epub 2025 Jun 23. Anal Chem. 2025. PMID: 40551446 Free PMC article.
-
Thermo-responsive imprinted hydrogel with switchable sialic acid recognition for selective cancer cell isolation from blood.Bioact Mater. 2020 Nov 9;6(5):1308-1317. doi: 10.1016/j.bioactmat.2020.10.008. eCollection 2021 May. Bioact Mater. 2020. PMID: 33251380 Free PMC article.
References
-
- Turner A.P.F. Biosensors: sense and sensibility. Chem. Soc. Rev. 2013;42:3184–3196. - PubMed
-
- Lei J.P., Ju H.X. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012;41:2122–2134. - PubMed
-
- Yang G., Xiao Z., Tang C., Deng Y., Huang H., He Z. Recent advances in biosensor for detection of lung cancer biomarkers. Biosens. Bioelectron. 2019;141:111416. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
