Ensuring both velocity and spatial responses robust to field inhomogeneities for velocity-selective arterial spin labeling through dynamic phase-cycling
- PMID: 33349968
- PMCID: PMC7962600
- DOI: 10.1002/mrm.28622
Ensuring both velocity and spatial responses robust to field inhomogeneities for velocity-selective arterial spin labeling through dynamic phase-cycling
Abstract
Purpose: To evaluate both velocity and spatial responses of velocity-selective arterial spin labeling (VS-ASL), using velocity-insensitive and velocity-compensated waveforms for control modules, as well as a novel dynamic phase-cycling approach, at different B0 / field inhomogeneities.
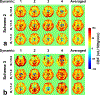
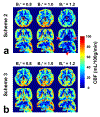
Methods: In the presence of imperfect refocusing, the mechanism of phase-cycling the refocusing pulses through four dynamics was first theoretically analyzed with the conventional velocity-selective saturation (VSS) pulse train. Numerical simulations were then deployed to compare the performance of the Fourier-transform based velocity-selective inversion (FT-VSI) with these three different schemes in terms of both velocity and spatial responses under various B0 / conditions. Phantom and human brain scans were performed to evaluate the three methods at scales of 0.8, 1.0, and 1.2.
Results: The simulations of FT-VSI showed that, under nonuniform B0 / conditions, the scheme with velocity-insensitive control was susceptible to DC bias of the static spins as systematic error, while the scheme with velocity-compensated control had deteriorated velocity-selective labeling profiles and, thus, reduced labeling efficiency. Through numerical simulation, phantom scans, and brain perfusion measurements, the dynamic phase-cycling method demonstrated considerable improvements over these issues.
Conclusion: The proposed dynamic phase-cycling approach was demonstrated for the velocity-selective label and control modules with both velocity and spatial responses robust to a wide range of B0 and field inhomogeneities.
Keywords: field inhomogeneity; B0 field inhomogeneity; arterial spin labeling; cerebral blood flow; velocity-selective inversion.
© 2020 International Society for Magnetic Resonance in Medicine.
Figures





References
-
- Wong EC, Cronin M, Wu WC, Inglis B, Frank LR, Liu TT. Velocity-selective arterial spin labeling. Magn. Reson. Med 2006;55:1334–1341. - PubMed
-
- Duhamel G, de Bazelaire C, Alsop DC. Evaluation of systematic quantification errors in velocity-selective arterial spin labeling of the brain. Magn. Reson. Med 2003;50:145–153. - PubMed
-
- Wu WC, Wong EC. Intravascular effect in velocity-selective arterial spin labeling: The choice of inflow time and cutoff velocity. Neuroimage 2006;32:122–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

