Muscular changes in animal models of heart failure with preserved ejection fraction: what comes closest to the patient?
- PMID: 33350094
- PMCID: PMC7835579
- DOI: 10.1002/ehf2.13142
Muscular changes in animal models of heart failure with preserved ejection fraction: what comes closest to the patient?
Abstract
Aims: Heart failure with preserved ejection fraction (HFpEF) is associated with reduced exercise capacity elicited by skeletal muscle (SM) alterations. Up to now, no clear medical treatment advice for HFpEF is available. Identification of the ideal animal model mimicking the human condition is a critical step in developing and testing treatment strategies. Several HFpEF animals have been described, but the most suitable in terms of comparability with SM alterations in HFpEF patients is unclear. The aim of the present study was to investigate molecular changes in SM of three different animal models and to compare them with alterations of muscle biopsies obtained from human HFpEF patients.
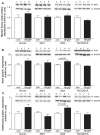
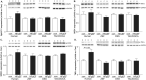
Methods and results: Skeletal muscle tissue was obtained from HFpEF and control patients and from three different animal models including the respective controls-ZSF1 rat, Dahl salt-sensitive rat, and transverse aortic constriction surgery/deoxycorticosterone mouse. The development of HFpEF was verified by echocardiography. Protein expression and enzyme activity of selected markers were assessed in SM tissue homogenates. Protein expression between SM tissue obtained from HFpEF patients and the ZSF1 rats revealed similarities for protein markers involved in muscle atrophy (MuRF1 expression, protein ubiquitinylation, and LC3) and mitochondrial metabolism (succinate dehydrogenase and malate dehydrogenase activity, porin expression). The other two animal models exhibited far less similarities to the human samples.
Conclusions: None of the three tested animal models mimics the condition in HFpEF patients completely, but among the animal models tested, the ZSF1 rat (ZSF1-lean vs. ZSF1-obese) shows the highest overlap to the human condition. Therefore, when studying therapeutic interventions to treat HFpEF and especially alterations in the SM, we suggest that the ZSF1 rat is a suitable model.
Keywords: Animal models; Heart failure with preserved ejection fraction; Skeletal muscle.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Ephraim Winzer reports personal fees from Boehringer‐Ingelheim, Novartis, and CVRx outside of this study.
Figures



Similar articles
-
ZSF1 rat as animal model for HFpEF: Development of reduced diastolic function and skeletal muscle dysfunction.ESC Heart Fail. 2020 Oct;7(5):2123-2134. doi: 10.1002/ehf2.12915. Epub 2020 Jul 25. ESC Heart Fail. 2020. PMID: 32710530 Free PMC article.
-
Targeting MuRF1 by small molecules in a HFpEF rat model improves myocardial diastolic function and skeletal muscle contractility.J Cachexia Sarcopenia Muscle. 2022 Jun;13(3):1565-1581. doi: 10.1002/jcsm.12968. Epub 2022 Mar 17. J Cachexia Sarcopenia Muscle. 2022. PMID: 35301823 Free PMC article.
-
Exercise Training Reveals Inflexibility of the Diaphragm in an Animal Model of Patients With Obesity-Driven Heart Failure With a Preserved Ejection Fraction.J Am Heart Assoc. 2017 Oct 24;6(10):e006416. doi: 10.1161/JAHA.117.006416. J Am Heart Assoc. 2017. PMID: 29066440 Free PMC article.
-
Clinical Phenotypes of Heart Failure With Preserved Ejection Fraction to Select Preclinical Animal Models.JACC Basic Transl Sci. 2022 May 25;7(8):844-857. doi: 10.1016/j.jacbts.2021.12.009. eCollection 2022 Aug. JACC Basic Transl Sci. 2022. PMID: 36061340 Free PMC article. Review.
-
Skeletal muscle abnormalities in heart failure with preserved ejection fraction.Heart Fail Rev. 2023 Jan;28(1):157-168. doi: 10.1007/s10741-022-10219-9. Epub 2022 Mar 30. Heart Fail Rev. 2023. PMID: 35353269 Review.
Cited by
-
Characteristics and outcomes of heart failure with recovered left ventricular ejection fraction.ESC Heart Fail. 2021 Dec;8(6):5383-5391. doi: 10.1002/ehf2.13630. Epub 2021 Sep 27. ESC Heart Fail. 2021. PMID: 34569712 Free PMC article.
-
Modulation of Titin and Contraction-Regulating Proteins in a Rat Model of Heart Failure with Preserved Ejection Fraction: Limb vs. Diaphragmatic Muscle.Int J Mol Sci. 2024 Jun 16;25(12):6618. doi: 10.3390/ijms25126618. Int J Mol Sci. 2024. PMID: 38928324 Free PMC article.
-
Empagliflozin Preserves Skeletal Muscle Function in a HFpEF Rat Model.Int J Mol Sci. 2022 Sep 20;23(19):10989. doi: 10.3390/ijms231910989. Int J Mol Sci. 2022. PMID: 36232292 Free PMC article.
-
Heart Failure with Preserved Ejection Fraction: Pathogenesis, Diagnosis, Exercise, and Medical Therapies.J Cardiovasc Transl Res. 2023 Apr;16(2):310-326. doi: 10.1007/s12265-022-10324-y. Epub 2022 Sep 28. J Cardiovasc Transl Res. 2023. PMID: 36171526 Review.
-
Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction.Int J Mol Sci. 2025 Jun 27;26(13):6196. doi: 10.3390/ijms26136196. Int J Mol Sci. 2025. PMID: 40649974 Free PMC article.
References
-
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New England Journal of Medicine 2006; 355: 251–259. - PubMed
-
- Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017; 70: 2476–2486. - PubMed
-
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. New England Journal of Medicine 2006; 355: 260–269. - PubMed
-
- Nichols GA, Reynolds K, Kimes TM, Rosales AG, Chan WW. Comparison of risk of re‐hospitalization, all‐cause Mortality, and Medical Care Resource Utilization in Patients With Heart Failure and Preserved Versus Reduced Ejection Fraction. Am J Cardiol 2015; 116: 1088–1092. - PubMed
-
- Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction. Circulation 2012; 126: 65–75. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

