Validation of International Working Group response criteria in higher-risk myelodysplastic syndromes: A report on behalf of the MDS Clinical Research Consortium
- PMID: 33350168
- PMCID: PMC7877342
- DOI: 10.1002/cam4.3608
Validation of International Working Group response criteria in higher-risk myelodysplastic syndromes: A report on behalf of the MDS Clinical Research Consortium
Abstract
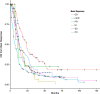
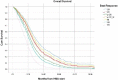
The utility of the International Working Group (IWG) 2006 response criteria for myelodysplastic syndromes (MDS) as a surrogate endpoint for outcomes is unclear. We assessed the validity of the IWG 2006 response criteria in a large cohort of higher-risk MDS patients (pts) treated at centers from the MDS Clinical Research Consortium. The best overall response rate (ORR) by IWG 2006 criteria to first-line therapy among 597 evaluable pts was 38% and include complete response (CR) 16%, marrow CR (mCR) 2%, partial response (PR) 10%, hematological improvement (HI) 10%, stable disease (SD) 33%, and progressive disease (PD) 24%. CR was associated with a better overall survival (OS) compared to all other response groups (P < 0.001). Among 470 pts treated with hypomethylating agent (HMA) as first-line therapy, the overall Response Rate, defined as HI or better was 39%. The median OS from time of best response was 21 mo, 8 mo, 14 mo, 12 mo, 13 mo, and 8 mo for CR, mCR, PR, HI, SD, and PD, respectively (P < 0.001). We validated those results in a separate cohort of 539 higher-risk MDS pts treated at Moffitt Cancer Center who received first-line HMA therapy, particularly addressing the value of mCR and mCR+HI. mCR alone without HI, SD, and PD outcomes were inferior to CR, PR, mCR+HI, and HI. In conclusion, CR by IWG 2006 response criteria can be used as a surrogate endpoint for OS in higher-risk MDS pts. Any response associated with restoration of effective hematopoiesis is associated with better outcome.
Keywords: high-risk disease; international working group; myelodysplastic syndromes; response criteria.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Komrokji:
Figures
References
-
- Zeidan AM, Gore SD, Padron E, et al. Current state of prognostication and risk stratification in myelodysplastic syndromes. Curr Opin Hematol. 2015;22(2):146‐154. - PubMed
-
- Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671‐3674. - PubMed
-
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419‐425. - PubMed
-
- Gore SD, Fenaux P, Santini V, et al. A multivariate analysis of the relationship between response and survival among patients with higher‐risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA‐001 trial. Haematologica. 2013;98(7):1067‐1072. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous