Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses
- PMID: 33350337
- PMCID: PMC7832006
- DOI: 10.1080/22221751.2020.1868274
Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses
Abstract
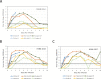
Strategies to control spread of highly pathogenic avian influenza (HPAI) viruses by wild birds appear limited, hence timely characterization of novel viruses is important to mitigate the risk for the poultry sector and human health. In this study we characterize three recent H5-clade 2.3.4.4 viruses, the H5N8-2014 group A virus and the H5N8-2016 and H5N6-2017 group B viruses. The pathogenicity of the three viruses for chickens, Pekin ducks and Eurasian wigeons was compared. The three viruses were highly pathogenic for chickens, but the two H5N8 viruses caused no to mild clinical symptoms in both duck species. The highest pathogenicity for duck species was observed for the most recent H5N6-2017 virus. For both duck species, virus shedding from the cloaca was higher after infection with group B viruses compared to the H5N8-2014 group A virus. Higher cloacal virus shedding of wild ducks may increase transmission between wild birds and poultry. Environmental transmission of H5N8-2016 virus to chickens was studied, which showed that chickens are efficiently infected by (fecal) contaminated water. These results suggest that pathogenicity of HPAI H5 viruses and virus shedding for ducks is evolving, which may have implications for the risk of introduction of these viruses into the poultry sector.
Keywords: Eurasian wigeons; H5N6; H5N8; Highly pathogenic avian influenza; ducks; environmental transmission; pathogenicity; virus shedding.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
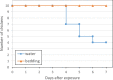
Figures


References
-
- Ramey AM, Reeves AB, TeSlaa JL, et al. . Evidence for common ancestry among viruses isolated from wild birds in Beringia and highly pathogenic intercontinental reassortant H5N1 and H5N2 influenza A viruses. Infect Genet Evol J Molec Epidemiol Evolution Genet Infect Dis. 2016;40:176–185. doi:10.1016/j.meegid.2016.02.035 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
