Determinants of Malaria Protective Immunity in Mice Immunized with Live Sporozoites during Trimethoprim-Sulfamethoxazole Prophylaxis
- PMID: 33350377
- PMCID: PMC7866335
- DOI: 10.4269/ajtmh.20-0749
Determinants of Malaria Protective Immunity in Mice Immunized with Live Sporozoites during Trimethoprim-Sulfamethoxazole Prophylaxis
Abstract
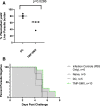
HIV and malaria geographically overlap. Trimethoprim-sulfamethoxazole (TMP-SMX) is a drug widely used in HIV-exposed uninfected and infected children in malaria-endemic areas, and is known to have antimalarial effects. Further study in terms of antimalarial impact and effect on development of malaria-specific immunity is therefore essential. Using rodent malaria models, we previously showed that repeated Plasmodium exposure during TMP-SMX administration, or chemoprophylaxis vaccination (CVac), induces CD8 T-cell-dependent preerythrocytic immunity. However, humoral immune responses have been shown to be important in models of preerythrocytic immunity. Herein, we demonstrate that antibody-mediated responses contribute to protective immunity induced by CVac immune sera using TMP-SMX in models of homologous, but not heterologous, parasite species. Clinical studies must account for potential anti-Plasmodium antibody induced during TMP-SMX prophylaxis.
Conflict of interest statement
Disclosure: The study received support from the Animal Team at Laboratory of Malaria Immunology and Vaccinology (Yvette Robbins and Lynn Lambert), the Insectary Management Team at Laboratory of Malaria and Vector Research (Kevin Spinno and Andre Laughinghouse), and the Animal Husbandry Unit (Jade Basil, Christopher Brown, April Cory, Melissa Krefski, Andrew Popadich, Megan Snyder, and Lisa Torzewsku, Marsha Loll) at the NIH.
Figures
References
-
- Gregson A, Plowe CV, 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev 57: 117–145. - PubMed
-
- Church JA, Fitzgerald F, Walker AS, Gibb DM, Prendergast AJ, 2015. The expanding role of co-trimoxazole in developing countries. Lancet Infect Dis 15: 327–339. - PubMed
-
- Homsy J, Dorsey G, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, Muhindo M, Kamya MR, Sandison TG, Tappero JW, 2014. Protective efficacy of prolonged co-trimoxazole prophylaxis in HIV-exposed children up to age 4 years for the prevention of malaria in Uganda: a randomised controlled open-label trial. Lancet Glob Health 2: e727–e736. - PubMed
-
- Nussenzweig RS, Vanderberg JP, Most H, Orton C, 1969. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature 222: 488–489. - PubMed
-
- Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K, 2009. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J Infect Dis 199: 899–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

