Cellular quiescence in budding yeast
- PMID: 33350503
- PMCID: PMC8208048
- DOI: 10.1002/yea.3545
Cellular quiescence in budding yeast
Abstract
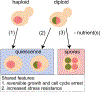
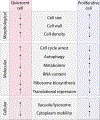
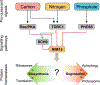
Cellular quiescence, the temporary and reversible exit from proliferative growth, is the predominant state of all cells. However, our understanding of the biological processes and molecular mechanisms that underlie cell quiescence remains incomplete. As with the mitotic cell cycle, budding and fission yeast are preeminent model systems for studying cellular quiescence owing to their rich experimental toolboxes and the evolutionary conservation across eukaryotes of pathways and processes that control quiescence. Here, we review current knowledge of cell quiescence in budding yeast and how it pertains to cellular quiescence in other organisms, including multicellular animals. Quiescence entails large-scale remodeling of virtually every cellular process, organelle, gene expression, and metabolic state that is executed dynamically as cells undergo the initiation, maintenance, and exit from quiescence. We review these major transitions, our current understanding of their molecular bases, and highlight unresolved questions. We summarize the primary methods employed for quiescence studies in yeast and discuss their relative merits. Understanding cell quiescence has important consequences for human disease as quiescent single-celled microbes are notoriously difficult to kill and quiescent human cells play important roles in diseases such as cancer. We argue that research on cellular quiescence will be accelerated through the adoption of common criteria, and methods, for defining cell quiescence. An integrated approach to studying cell quiescence, and a focus on the behavior of individual cells, will yield new insights into the pathways and processes that underlie cell quiescence leading to a more complete understanding of the life cycle of cells. TAKE AWAY: Quiescent cells are viable cells that have reversibly exited the cell cycle Quiescence is induced in response to a variety of nutrient starvation signals Quiescence is executed dynamically through three phases: initiation, maintenance, and exit Quiescence entails large-scale remodeling of gene expression, organelles, and metabolism Single-cell approaches are required to address heterogeneity among quiescent cells.
© 2020 John Wiley & Sons, Ltd.
Figures

Similar articles
-
The budding yeast transition to quiescence.Yeast. 2021 Jan;38(1):30-38. doi: 10.1002/yea.3546. Epub 2021 Jan 8. Yeast. 2021. PMID: 33350501
-
Mechanisms that Link Chronological Aging to Cellular Quiescence in Budding Yeast.Int J Mol Sci. 2020 Jul 2;21(13):4717. doi: 10.3390/ijms21134717. Int J Mol Sci. 2020. PMID: 32630624 Free PMC article. Review.
-
"Sleeping beauty": quiescence in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2004 Jun;68(2):187-206. doi: 10.1128/MMBR.68.2.187-206.2004. Microbiol Mol Biol Rev. 2004. PMID: 15187181 Free PMC article. Review.
-
Metabolic status rather than cell cycle signals control quiescence entry and exit.J Cell Biol. 2011 Mar 21;192(6):949-57. doi: 10.1083/jcb.201009028. Epub 2011 Mar 14. J Cell Biol. 2011. PMID: 21402786 Free PMC article.
-
Leo1 is essential for the dynamic regulation of heterochromatin and gene expression during cellular quiescence.Epigenetics Chromatin. 2019 Jul 17;12(1):45. doi: 10.1186/s13072-019-0292-7. Epigenetics Chromatin. 2019. PMID: 31315658 Free PMC article.
Cited by
-
LEO1 Is Required for Efficient Entry into Quiescence, Control of H3K9 Methylation and Gene Expression in Human Fibroblasts.Biomolecules. 2023 Nov 17;13(11):1662. doi: 10.3390/biom13111662. Biomolecules. 2023. PMID: 38002344 Free PMC article.
-
Triosephosphate Isomerase and Its Product Glyceraldehyde-3-Phosphate Are Involved in the Regulatory Mechanism That Suppresses Exit from the Quiescent State in Yeast Cells.Microbiol Spectr. 2022 Aug 31;10(4):e0089722. doi: 10.1128/spectrum.00897-22. Epub 2022 Aug 4. Microbiol Spectr. 2022. PMID: 35924934 Free PMC article.
-
Diverse geroprotectors differently affect a mechanism linking cellular aging to cellular quiescence in budding yeast.Oncotarget. 2022 Jul 28;13:918-943. doi: 10.18632/oncotarget.28256. eCollection 2022. Oncotarget. 2022. PMID: 35937500 Free PMC article.
-
Cyclin C-Cdk8 Kinase Phosphorylation of Rim15 Prevents the Aberrant Activation of Stress Response Genes.Front Cell Dev Biol. 2022 Mar 31;10:867257. doi: 10.3389/fcell.2022.867257. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433688 Free PMC article.
-
Transcriptional Shift and Metabolic Adaptations during Leishmania Quiescence Using Stationary Phase and Drug Pressure as Models.Microorganisms. 2022 Jan 3;10(1):97. doi: 10.3390/microorganisms10010097. Microorganisms. 2022. PMID: 35056546 Free PMC article.
References
-
- Alexander BD, & Perfect JR (1997). Antifungal resistance trends towards the year 2000. Drugs, 54(5), 657–678. - PubMed
-
- Argüello-Miranda O, Liu Y, Wood NE, Kositangool P, & Doncic A (2018). Integration of Multiple Metabolic Signals Determines Cell Fate Prior to Commitment. Molecular Cell, 71(5), 733–744.e11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
