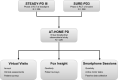
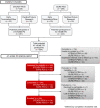
Design of a virtual longitudinal observational study in Parkinson's disease (AT-HOME PD)
- PMID: 33350601
- PMCID: PMC7886038
- DOI: 10.1002/acn3.51236
Design of a virtual longitudinal observational study in Parkinson's disease (AT-HOME PD)
Abstract
Objective: The expanding power and accessibility of personal technology provide an opportunity to reduce burdens and costs of traditional clinical site-centric therapeutic trials in Parkinson's disease and generate novel insights. The value of this approach has never been more evident than during the current COVID-19 pandemic. We sought to (1) establish and implement the infrastructure for longitudinal, virtual follow-up of clinical trial participants, (2) compare changes in smartphone-based assessments, online patient-reported outcomes, and remote expert assessments, and (3) explore novel digital markers of Parkinson's disease disability and progression.
Methods: Participants from two recently completed phase III clinical trials of inosine and isradipine enrolled in Assessing Tele-Health Outcomes in Multiyear Extensions of Parkinson's Disease trials (AT-HOME PD), a two-year virtual cohort study. After providing electronic informed consent, individuals complete annual video visits with a movement disorder specialist, smartphone-based assessments of motor function and socialization, and patient-reported outcomes online.
Results: From the two clinical trials, 226 individuals from 42 states in the United States and Canada enrolled. Of these, 181 (80%) have successfully downloaded the study's smartphone application and 161 (71%) have completed patient-reported outcomes on the online platform.
Interpretation: It is feasible to conduct a large-scale, international virtual observational study following the completion of participation in brick-and-mortar clinical trials in Parkinson's disease. This study, which brings research to participants, will compare established clinical endpoints with novel digital biomarkers and thereby inform the longitudinal follow-up of clinical trial participants and design of future clinical trials.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Ruth B Schneider, MD – Dr. Schneider reports grants from Acadia Pharmaceuticals, Biohaven Pharmaceuticals, The Michael J. Fox Foundation for Parkinson’s Research, NIH/NINDS, and CHDI outside the submitted work. Larsson Omberg, PhD – Dr. Omberg was an employee of Sage Bionetworks during the conduct of the study and report grants from The Michael J. Fox Foundation for Parkinson’s Research, Novartis Pharmaceuticals, Celgene, NIH/NIA, NIH/NIMH, NIH/NINDS, Alzheimer's Drug Discovery Foundation, The Gates Foundation, The Wellcome Trust, and The Leona M. and Harry B. Helmsley Charitable Trust outside the submitted work. Eric A Macklin, PhD – Dr. Macklin reports grants from NIH/NINDS during the conduct of the study; grants from Acorda Therapeutics, Amylyx Pharmaceuticals, GlaxoSmithKline, and Mitsubishi Tanabe Pharmaceuticals; and other from Biogen, Cerevance, Acorda Therapeutics, Novartis Pharmaceuticals, Shire Human Genetic Therapies, and Stoparkinson Healthcare Systems outside the submitted work. Margaret Daeschler, BA – Ms. Daeschler was an employee of the Michael J. Fox Foundation during the conduct of the study. Lauren Bataille, MS – Ms. Bataille has nothing to disclose. Shalini Anthwal, PhD, MSc – Dr. Anthwal reports grants from NINDS during the conduct of the study. Taylor L Myers, BA – Ms. Myers reports grants from NINDS during the conduct of the study. Elizabeth Baloga, MPH – M. Baloga reports grants from NIH/NINDS and nonfinancial support from the Michael J. Fox Foundation for Parkinson's Research during the conduct of the study; grants from the Michael J. Fox Foundation for Parkinson's Research, and nonfinancial support from Parkinson's Foundation outside the submitted work. Sidney Duquette, BS – Mr. Duquette has nothing to disclose. Phil Snyder, BS – Mr. Snyder has nothing to disclose. Katherine Amodeo, MD – Dr. Amodeo was supported by the Michael J Fox Edmund J. Safra Fellowship during 2017‐2019. Outside the submitted work, KA has served as co‐investigator or medical monitor for clinical trials supported by Genentech Roche Ltd, EIP Pharma Inc, The Michael J Fox foundation for PD research, NINDS, Acadia Pharmaceuticals Inc, and Biogene. Christopher G Tarolli, MD – Dr. Tarolli has nothing to disclose. Jamie L Adams, MD – Dr. Adams reports grants from Biogen, National Institutes of Health/National Institute of Neurological Disorders and Stroke, and The Michael J. Fox Foundation for Parkinson’s Research outside the submitted work. Katherine F Callahan, BS – Ms. Callahan reports grants from NIH during the conduct of the study. Joshua Gottesman, MA – Mr. Gottesman has nothing to disclose. Catherine M Kopil, PhD – Dr. Kopil reports sub‐award on NIH grant, and other from The Michael J. Fox Foundation for Parkinson's Research during the conduct of the study. Codrin Lungu, MD – Dr. Lungu has nothing to disclose. Alberto Ascherio, MD, DrPH – Dr. Ascherio has nothing to disclose. James C Beck, PhD – Dr. Beck has nothing to disclose. Kevin Biglan, MD, MPH – Dr. Biglan reports no disclosures other than Eli Lilly outside the submitted work. Alberto J Espay, MD, MSc ‐ Dr. Espay reports grants from NIH, Great Lakes Neurotechnologies, and The Michael J Fox Foundation, and personal fees from Abbvie, TEVA, Impax, Acadia, Acorda, Cynapsus/Sunovion, Lundbeck, US WorldMeds, UCB, Lippincott Williams & Wilkins, Cambridge University Press, and Springer outside the submitted work. Caroline Tanner, MD, PhD – Dr. Tanner reports grants from The Michael J Fox Foundation during the conduct of the study; grants from Parkinson Foundation, Gateway LLC, Roche/Genentech, The Michael J Fox Foundation, NIH/NIA, NIH/NINDS, VA Merit, The U.S. Department of Defense, Biogen Idec and Parkinson Study Group; personal fees from Biogen Idec, Acorda, Adamas Therapeutics, Amneal, CNS Ratings, Grey Matter LLC, Northwestern University, Partners, Guidemark Health, Acadia, Neurocrine, Lundbeck, Cadent, and Harvard University; and nonfinancial support from Medtronic, Inc., Acadia, Boston Scientific, Neurocrine, and Biogen Idec outside the submitted work. David Oakes, PhD – Dr. Oakes has nothing to disclose. Ira Shoulson, MD – Dr. Shoulson reports other from Grey Matter Technologies (GMT), Biogen, and Jazz Pharmaceuticals and grants from The Michael J Fox Foundation for Parkinson's Research outside the submitted work. Dan Novak, PhD – Dr. Novak has nothing to disclose. Elise Kayson, MS – Ms. Kayson has nothing to disclose. E. Ray Dorsey, MD ‐‐ Dr. Dorsey reports grants from NINDS during the conduct of the study; grants from Burroughs Wellcome Fund, Davis Phinney Foundation, Food and Drug Administration, Greater Rochester Health Foundation, Huntington Study Group, The Michael J. Fox Foundation, National Science Foundation, Patient‐Centered Outcomes Research Institute, Safra Foundation, grants from Duke University, University of California Irvine, grants from Abbvie, Acadia Pharmaceuticals, AMC Health, BioSensics, GlaxoSmithKline, Nuredis Pharmaceuticals, Pfizer, Prana Biotechnology, Raptor Pharmaceuticals, Roche, Teva Pharmaceuticals, personal fees from 23 and Me, Abbott, Abbvie, American Well, Biogen, BrainNeuroBio, Clintrex, Curasen Therapeutics, DeciBio, Denali Therapeutics, GlaxoSmithKline, Grand Rounds, Karger, Lundbeck, MC10, MedAvante, Medical‐legal services, Mednick Associates, National Institute of Neurological Disorders and Stroke, Olson Research Group, Optio, Origent Data Sciences, Inc., Otsuka, Prilenia, Putnam Associates, Roche, Sanofi, Shire, Spark, Sunovion Pharma, Teva, Theravance, UCB and Voyager Therapeutics, outside the submitted work; editorial services for Karger Publications; and ownership interests with Blackfynn and Grand Rounds. Lara Mangravite, PhD ‐‐ Dr. Mangravite reports grants from the National Institute of Health during the conduct of the study; grants from Novartis, Genentech/Roche, Celgene, and BMS outside the submitted work. Michael A Schwarzschild, MD, PhD – Dr. Schwarzschild reports grants from NIH during the conduct of the study. Tanya Simuni, MD – Dr. Simuni reports grants from NINDS, The Michael J Fox Foundation, Parkinson’s Foundation, Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Abbvie, IMPAX, and Prevail; other from Acadia, Abbvie, Accorda, Adamas, Allergan, Amneal, Aptinyx, Denali, General Electric (GE), Kyowa, Neuroderm, Neurocrine, Sanofi, Sinopia, Sunovion, Roche, Takeda, Voyager, and U.S. World Meds during the conduct of the study.
Figures
References
-
- Tufts Center for the Study of Drug Development . CNS Drugs Take Longer to Develop and Have Lower Success Rates than Other. 2014.
-
- Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. - PubMed
-
- Parkinson Study Group QEI , Beal MF, Oakes D, et al. A randomized clinical trial of high‐dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71:543–552. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical