En Route to the Transformation of Glycoscience: A Chemist's Perspective on Internal and External Crossroads in Glycochemistry
- PMID: 33350830
- PMCID: PMC7856254
- DOI: 10.1021/jacs.0c11106
En Route to the Transformation of Glycoscience: A Chemist's Perspective on Internal and External Crossroads in Glycochemistry
Abstract
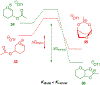
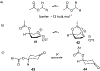
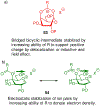
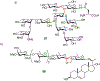
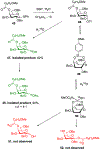
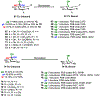
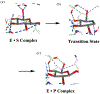
Carbohydrate chemistry is an essential component of the glycosciences and is fundamental to their progress. This Perspective takes the position that carbohydrate chemistry, or glycochemistry, has reached three crossroads on the path to the transformation of the glycosciences, and illustrates them with examples from the author's and other laboratories. The first of these potential inflexion points concerns the mechanism of the glycosylation reaction and the role of protecting groups. It is argued that the experimental evidence supports bimolecular SN2-like mechanisms for typical glycosylation reactions over unimolecular ones involving stereoselective attack on naked glycosyl oxocarbenium ions. Similarly, it is argued that the experimental evidence does not support long-range stereodirecting participation of remote esters through bridged bicyclic dioxacarbenium ions in organic solution in the presence of typical counterions. Rational design and improvement of glycosylation reactions must take into account the roles of the counterion and of concentration. A second crossroads is that between mainstream organic chemistry and glycan synthesis. The case is made that the only real difference between glycan and organic synthesis is the formation of C-O rather than C-C bonds, with diastereocontrol, strategy, tactics, and elegance being of critical importance in both areas: mainstream organic chemists should feel comfortable taking this fork in the road, just as carbohydrate chemists should traveling in the opposite direction. A third crossroads is that between carbohydrate chemistry and medicinal chemistry, where there are equally many opportunities for traffic in either direction. The glycosciences have advanced enormously in the past decade or so, but creativity, input, and ingenuity of scientists from all fields is needed to address the many sophisticated challenges that remain, not the least of which is the development of a broader and more general array of stereospecific glycosylation reactions.
Figures


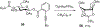

Similar articles
-
Glycosyl Oxocarbenium Ions: Structure, Conformation, Reactivity, and Interactions.Acc Chem Res. 2021 Jun 1;54(11):2552-2564. doi: 10.1021/acs.accounts.1c00021. Epub 2021 Apr 30. Acc Chem Res. 2021. PMID: 33930267 Free PMC article. Review.
-
Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity.Acc Chem Res. 2018 Mar 20;51(3):628-639. doi: 10.1021/acs.accounts.7b00449. Epub 2018 Feb 22. Acc Chem Res. 2018. PMID: 29469568 Review.
-
Direct Experimental Characterization of a Bridged Bicyclic Glycosyl Dioxacarbenium Ion by 1 H and 13 C NMR Spectroscopy: Importance of Conformation on Participation by Distal Esters.Angew Chem Int Ed Engl. 2021 Nov 22;60(48):25397-25403. doi: 10.1002/anie.202110212. Epub 2021 Oct 21. Angew Chem Int Ed Engl. 2021. PMID: 34543505 Free PMC article.
-
Mechanistic Investigations into the Application of Sulfoxides in Carbohydrate Synthesis.Chemistry. 2016 Mar 14;22(12):3916-28. doi: 10.1002/chem.201503504. Epub 2016 Jan 7. Chemistry. 2016. PMID: 26744250 Free PMC article. Review.
-
Mechanism of a chemical glycosylation reaction.Acc Chem Res. 2010 Aug 17;43(8):1144-53. doi: 10.1021/ar100035r. Acc Chem Res. 2010. PMID: 20496888
Cited by
-
Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation.Nat Chem. 2022 Jun;14(6):686-694. doi: 10.1038/s41557-022-00918-z. Epub 2022 Apr 11. Nat Chem. 2022. PMID: 35410373
-
Competing C-4 and C-5-Acyl Stabilization of Uronic Acid Glycosyl Cations.Chemistry. 2022 Nov 11;28(63):e202201724. doi: 10.1002/chem.202201724. Epub 2022 Sep 12. Chemistry. 2022. PMID: 35959853 Free PMC article.
-
Side Chain Conformation and Its Influence on Glycosylation Selectivity in Hexo- and Higher Carbon Furanosides.J Org Chem. 2022 Jan 7;87(1):316-339. doi: 10.1021/acs.joc.1c02374. Epub 2021 Dec 14. J Org Chem. 2022. PMID: 34905382 Free PMC article.
-
Stereodirecting Effect of Esters at the 4-Position of Galacto- and Glucopyranosyl Donors: Effect of 4-C-Methylation on Side-Chain Conformation and Donor Reactivity, and Influence of Concentration and Stoichiometry on Distal Group Participation.J Org Chem. 2023 Oct 6;88(19):13883-13893. doi: 10.1021/acs.joc.3c01496. Epub 2023 Sep 7. J Org Chem. 2023. PMID: 37677151 Free PMC article.
-
Emerging Capabilities of Nonclassical Noncovalent Interactions and Asymmetric Catalysis in Stereoselective Glycosylations and Carbohydrate Functionalizations.Acc Chem Res. 2025 Jul 1;58(13):2124-2144. doi: 10.1021/acs.accounts.5c00289. Epub 2025 Jun 12. Acc Chem Res. 2025. PMID: 40503847 Free PMC article.
References
-
- Walt DR; Aoki-Kinoshita KF; Bendiak B; Bertozzi CR; Boons G-J; Darvill A; Hart GW; Kiessling LL; Lowe J; Moon R; Paulson J; Sasisekharan R; Varki AP; Wong C-H Transforming Glycoscience: A Roadmap for the Future (2012); National Research Council: Washinggton DC, 2012.
-
- Glycoscience. https://commonfund.nih.gov/Glycoscience.
-
- Crich D; Ritchie TJ, Stereoselective Free Radical Reactions in the Preparation of 2-Deoxy-β-D-glucosides. J. Chem. Soc., Chem. Commun 1988, 1461–1463.
-
- Winstein S; Clippinger E; Fainberg AH; Heck R; Robinson GC, Salt Effects and Ion Pairs in Solvolysis. Common Ion Rate Depression and Exchange of Anions During Acetolysis. J. Am. Chem. Soc 1956, 78, 328–335.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

