MRI-guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology
- PMID: 33350894
- PMCID: PMC7924409
- DOI: 10.1148/radiol.2020202747
MRI-guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology
Abstract
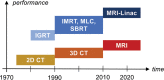
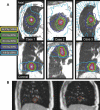
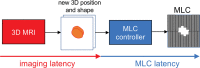
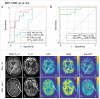
Radiation therapy (RT) continues to be one of the mainstays of cancer treatment. Considerable efforts have been recently devoted to integrating MRI into clinical RT planning and monitoring. This integration, known as MRI-guided RT, has been motivated by the superior soft-tissue contrast, organ motion visualization, and ability to monitor tumor and tissue physiologic changes provided by MRI compared with CT. Offline MRI is already used for treatment planning at many institutions. Furthermore, MRI-guided linear accelerator systems, allowing use of MRI during treatment, enable improved adaptation to anatomic changes between RT fractions compared with CT guidance. Efforts are underway to develop real-time MRI-guided intrafraction adaptive RT of tumors affected by motion and MRI-derived biomarkers to monitor treatment response and potentially adapt treatment to physiologic changes. These developments in MRI guidance provide the basis for a paradigm change in treatment planning, monitoring, and adaptation. Key challenges to advancing MRI-guided RT include real-time volumetric anatomic imaging, addressing image distortion because of magnetic field inhomogeneities, reproducible quantitative imaging across different MRI systems, and biologic validation of quantitative imaging. This review describes emerging innovations in offline and online MRI-guided RT, exciting opportunities they offer for advancing research and clinical care, hurdles to be overcome, and the need for multidisciplinary collaboration.
© RSNA, 2020.
Figures






References
-
- Chen AM, Hsu S, Lamb J, et al. MRI-guided radiotherapy for head and neck cancer: initial clinical experience. Clin Transl Oncol 2018;20(2):160–168. - PubMed
-
- Hunt A, Hansen VN, Oelfke U, Nill S, Hafeez S. Adaptive Radiotherapy Enabled by MRI Guidance. Clin Oncol (R Coll Radiol) 2018;30(11):711–719. - PubMed
-
- Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol 2014;24(3):196–199. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

