Impact of Human Papillomavirus Vaccination, Rwanda and Bhutan
- PMID: 33350922
- PMCID: PMC7774553
- DOI: 10.3201/eid2701.191364
Impact of Human Papillomavirus Vaccination, Rwanda and Bhutan
Abstract
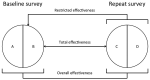
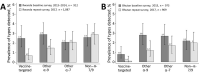
Rwanda and Bhutan, 2 low- and middle-income countries, implemented primarily school-based national human papillomavirus (HPV) vaccination in 2011 (Rwanda) and 2010 (Bhutan). We estimated vaccination effectiveness through urine-based HPV prevalence surveys in schools in 2013-2014 and 2017. In Rwanda, 912 participants from baseline surveys and 1,087 from repeat surveys were included, and in Bhutan, 973 participants from baseline surveys and 909 from repeat surveys were included. The overall effectiveness against vaccine-targeted HPV types (i.e., HPV-6/11/16/18) was 78% (95% CI 51%-90%) in Rwanda, and 88% (6%-99%) in Bhutan and against other α-9 types was 58% (21-78) in Rwanda and 63% (27-82) in Bhutan. No effect against other HPV types was detectable. Prevalence of vaccine-targeted HPV types decreased significantly, as well as that of other α-9 types, suggesting cross-protection. These findings provide direct evidence from low- and middle-income countries of the marked effectiveness of high-coverage school-based, national HPV vaccination programs.
Keywords: Bhutan; Rwanda; disease control; epidemiologic monitoring; human papillomavirus recombinant vaccine quadrivalent; low-income population; papillomavirus infections; papillomavirus vaccines; type 11; type 16; type 18; type 6; vaccine-preventable diseases; vaccines; viruses.
Figures

References
-
- World Health Organization. WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer. 2018. [cited 2019 Mar 19]. https://www.who.int/reproductivehealth/call-to-action-elimination-cervic...
-
- Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study. Lancet Oncol. 2019;20:394–407. 10.1016/S1470-2045(18)30836-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

