A 3D human adipose tissue model within a microfluidic device
- PMID: 33351023
- PMCID: PMC7876365
- DOI: 10.1039/d0lc00981d
A 3D human adipose tissue model within a microfluidic device
Abstract
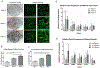
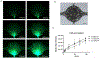
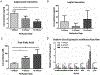
An accurate in vitro model of human adipose tissue could assist in the study of adipocyte function and allow for better tools for screening new therapeutic compounds. Cell culture models on two-dimensional surfaces fall short of mimicking the three-dimensional in vivo adipose environment, while three-dimensional culture models are often unable to support long-term cell culture due, in part, to insufficient mass transport. Microfluidic systems have been explored for adipose tissue models. However, current systems have primarily focused on 2D cultured adipocytes. In this work, a 3D human adipose microtissue was engineered within a microfluidic system. Human adipose-derived stem cells (ADSCs) were used as the cell source for generating differentiated adipocytes. The ADSCs differentiated within the microfluidic system formed a dense lipid-loaded mass with the expression of adipose tissue genetic markers. Engineered adipose tissue showed a decreased adiponectin secretion and increased free fatty acid secretion with increasing shear stress. Adipogenesis markers were downregulated with increasing shear stress. Overall, this microfluidic system enables the on-chip differentiation and development of a functional 3D human adipose microtissue supported by the interstitial flow. This system could potentially serve as a platform for in vitro drug testing for adipose tissue-related diseases.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures




References
-
- Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Bustos M, Martínez JA and Moreno-Aliaga MJ, Biochim. Biophys. Acta - Bioenerg, 2011, 1807, 664–678. - PubMed
-
- Percik R and Stumvoll M, Exp. Clin. Endocrinol. Diabetes, 2009, 117, 563–566. - PubMed
-
- Ungefroren H, Gieseler F, Fliedner S and Lehnert H, Horm. Mol. Biol. Clin. Investig, 2015, 21, 5–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

