The evolution of factor XI and the kallikrein-kinin system
- PMID: 33351111
- PMCID: PMC7757006
- DOI: 10.1182/bloodadvances.2020002456
The evolution of factor XI and the kallikrein-kinin system
Abstract
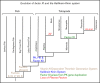
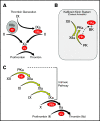
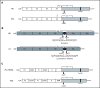
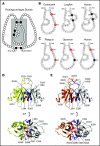
Factor XI (FXI) is the zymogen of a plasma protease (FXIa) that contributes to hemostasis by activating factor IX (FIX). In the original cascade model of coagulation, FXI is converted to FXIa by factor XIIa (FXIIa), a component, along with prekallikrein and high-molecular-weight kininogen (HK), of the plasma kallikrein-kinin system (KKS). More recent coagulation models emphasize thrombin as a FXI activator, bypassing the need for FXIIa and the KKS. We took an evolutionary approach to better understand the relationship of FXI to the KKS and thrombin generation. BLAST searches were conducted for FXI, FXII, prekallikrein, and HK using genomes for multiple vertebrate species. The analysis shows the KKS appeared in lobe-finned fish, the ancestors of all land vertebrates. FXI arose later from a duplication of the prekallikrein gene early in mammalian evolution. Features of FXI that facilitate efficient FIX activation are present in all living mammals, including primitive egg-laying monotremes, and may represent enhancement of FIX-activating activity inherent in prekallikrein. FXI activation by thrombin is a more recent acquisition, appearing in placental mammals. These findings suggest FXI activation by FXIIa may be more important to hemostasis in primitive mammals than in placental mammals. FXI activation by thrombin places FXI partially under control of the vitamin K-dependent coagulation mechanism, reducing the importance of the KKS in blood coagulation. This would explain why humans with FXI deficiency have a bleeding abnormality, whereas those lacking components of the KKS do not.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

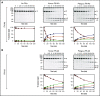
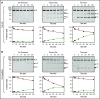
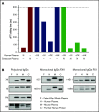
References
-
- Doolittle RF, Jiang Y, Nand J. Genomic evidence for a simpler clotting scheme in jawless vertebrates. J Mol Evol. 2008;66(2):185-196. - PubMed
-
- Doolittle RF. Step-by-step evolution of vertebrate blood coagulation. Cold Spring Harb Symp Quant Biol. 2009;74(0):35-40. - PubMed
-
- Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709-725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

