Intracranial hemorrhage with direct oral anticoagulants in patients with brain metastases
- PMID: 33351124
- PMCID: PMC7756985
- DOI: 10.1182/bloodadvances.2020003238
Intracranial hemorrhage with direct oral anticoagulants in patients with brain metastases
Abstract
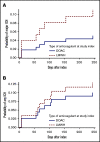
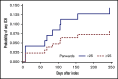
Direct oral anticoagulants (DOACs) are increasingly prescribed in treatment of cancer-associated thrombosis, but limited data exist regarding safety of DOACs in patients with brain metastases. We aimed to determine the incidence of intracranial hemorrhage (ICH) in patients with brain metastases receiving DOACs or low-molecular-weight heparin (LMWH) for venous thromboembolism or atrial fibrillation. An international 2-center retrospective cohort study was designed. Follow-up started on the first day of concomitant anticoagulation and brain tumor diagnosis. At least 2 brain imaging studies were mandated. The primary outcome was the cumulative incidence of any spontaneous ICH at 12-month follow-up with death as a competing risk. Major ICH was defined as spontaneous, ≥10 mL in volume, symptomatic, or requiring surgical intervention. Imaging studies were centrally reviewed by a neuroradiologist blinded for anticoagulant type. PANWARDS (platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, stroke) score for prediction of ICH was calculated. We included 96 patients with brain metastases (41 DOAC, 55 LMWH). The 12-month cumulative incidence of major ICH was 5.1% in DOAC-treated patients and 11.1% in those treated with LMWH (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.09-2.21). When anticoagulation was analyzed as a time-varying covariate, the risk of any ICH did not differ between DOAC- and LMWH-treated patients (HR, 0.98; 95% CI, 0.28-3.40). PANWARDS score was not associated with ICH risk. This international 2-center study suggests comparable safety of LMWH and DOACs in patients with brain metastases.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.L. reports personal fees from Pfizer and personal fees from Bayer Healthcare outside the submitted work. S.Y.-K. reports grants from BMS and personal fees from Novartis, AstraZeneca, and Teva outside the submitted work. S.M. reports grants and personal fees from Bayer, BMS Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, and Portola during the conduct of the study, as well as grants and personal fees from GSK and Aspen and personal fees from Sanofi outside the submitted work. H.R.B. reports grants and personal fees from Sanofi-Aventis, Bayer Healthcare, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, and Novartis outside the submitted work. J.I.Z. reports grants from Incyte during the conduct of the study and grants from Incyte and Quercegen, personal fees from CSL, Merck, Parexel, Sanofi, Daiichi, Pfizer/BMS, and Portola outside the submitted work. G.S. reports personal fees from Pfizer, Bayer, Boehringer Ingelheim, and Sanofi outside the submitted work. The remaining authors declare no competing financial interests.
Figures

References
-
- Erichsen R, Christiansen CF, Mehnert F, Weiss NS, Baron JA, Sørensen HT. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case-control study. Intern Emerg Med. 2012;7(5):431-438. - PubMed
-
- Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006;24(8):1310-1318. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

