Parenteral Lidocaine for Complex Cancer Pain in the Home or Inpatient Hospice Setting: A Review and Synthesis of the Evidence
- PMID: 33351710
- PMCID: PMC8309416
- DOI: 10.1089/jpm.2020.0622
Parenteral Lidocaine for Complex Cancer Pain in the Home or Inpatient Hospice Setting: A Review and Synthesis of the Evidence
Abstract
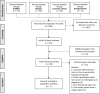
Background: Cancer pain can remain refractory despite escalating opioids and adjuvants. Systemic Lidocaine is an option, but current approaches are hospital centered. While advantageous in advanced cancer, evidence is lacking for parenteral Lidocaine use in community-based care. Objectives: Review evidence for parenteral lidocaine in complex cancer pain outside the hospital setting. Design: Systematic review of peer-reviewed articles of any study design, including reviews. Search in four databases used keyword variations of "cancer," "pain," "Lidocaine," and "parenteral." Search was extended through reference lists of full texts assessed. Abstracted data from articles screened and selected were synthesized narratively by a palliative care clinician in Singapore. Results: Eight hundred eighty-three articles identified were screened by title and abstract. Twenty-eight full texts were assessed. Seven articles fulfilled criteria for synthesis of findings. A total of 73 patients received parenteral Lidocaine for mixed pains, reported collectively in 1 retrospective chart review, 3 practice guidelines, 2 case series, and 1 case study. Intravenous or subcutaneous Lidocaine was commenced in hospital or hospice and continued at home. Dosages and administration schedules varied, involving slow bolus with continuous infusion or the latter alone, for up to 240 days. All produced positive outcomes, with no severe adverse events. Monitoring included routine vital signs and conscious levels; electrocardiogram, liver, and renal function tests were uncommon. Lidocaine levels were not consistently assessed. Conclusion: Parenteral Lidocaine can be effective and safe in the community setting. More empirical studies are needed to inform patient selection and treatment protocol, and to validate expected outcomes.
Keywords: cancer pain; home; hospice; palliative; parenteral Lidocaine.
Conflict of interest statement
No competing financial interests exist.
References
-
- Chia SC, Hum A, Ong WY, et al. : Parenteral lignocaine in cancer neuropathic pain: A series of case reports. Prog Palliat Care 2014;22:253–257
-
- Lee JT, Sanderson CR, Xuan W, et al. : Lidocaine for cancer pain in adults: A systematic review and meta-analysis. J Palliat Med 2019;22:326–334 - PubMed
-
- World Health Organization: WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: World Health Organization, 2018 - PubMed
-
- Thomas J, Kronenberg R, Cox MC, et al. : Intravenous lidocaine relieves severe pain: Results of an inpatient hospice chart review. J Palliat Med 2004;7:660–667 - PubMed
-
- Hawley P, Fyles G, Jefferys SG: Subcutaneous Lidocaine for cancer-related pain. J Palliat Med 2020;23:1357–1364 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical