Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis
- PMID: 33351782
- PMCID: PMC7934851
- DOI: 10.1172/jci.insight.140644
Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis
Abstract
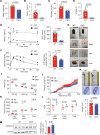
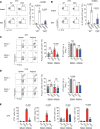
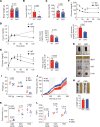
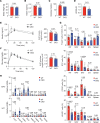
Interleukin-10 (IL-10) is a critical cytokine used by immune cells to suppress inflammation. Paradoxically, immune cell-derived IL-10 can drive insulin resistance in obesity by suppressing adipocyte energy expenditure and thermogenesis. However, the source of IL-10 necessary for the suppression of adipocyte thermogenesis is unknown. We show here that CD4+Foxp3+ regulatory T cells (Tregs) are a substantial source of IL-10 and that Treg-derived IL-10 can suppress adipocyte beiging. Unexpectedly, Treg-specific loss of IL-10 resulted in increased insulin sensitivity and reduced obesity in high-fat diet-fed male mice. Mechanistically, we determined that Treg-specific loss of the transcription factor Blimp-1, a driver of IL-10 expression by Tregs, phenocopied the Treg-specific IL-10-deficient mice. Loss of Blimp-1 expression in Tregs resulted in reduced ST2+KLRG1+, IL-10-secreting Tregs, particularly in the white adipose tissue. Blimp-1-deficient mice were protected from glucose intolerance, insulin resistance, and diet-induced obesity, through increased white adipose tissue browning. Taken together, our data show that Blimp-1-regulated IL-10 secretion by Tregs represses white adipose tissue beiging to maintain adipose tissue homeostasis.
Keywords: Adipose tissue; Cytokines; Endocrinology; Immunology; T cells.
Conflict of interest statement
Figures



References
-
- Villarroya F, et al. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 2018;27(5):954–961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

