HIV Rev-isited
- PMID: 33352061
- PMCID: PMC7776567
- DOI: 10.1098/rsob.200320
HIV Rev-isited
Abstract
The human immunodeficiency virus type 1 (HIV-1) proteome is expressed from alternatively spliced and unspliced genomic RNAs. However, HIV-1 RNAs that are not fully spliced are perceived by the host machinery as defective and are retained in the nucleus. During late infection, HIV-1 bypasses this regulatory mechanism by expression of the Rev protein from a fully spliced mRNA. Once imported into the nucleus, Rev mediates the export of unprocessed HIV-1 RNAs to the cytoplasm, leading to the production of the viral progeny. While regarded as a canonical RNA export factor, Rev has also been linked to HIV-1 RNA translation, stabilization, splicing and packaging. However, Rev's functions beyond RNA export have remained poorly understood. Here, we revisit this paradigmatic protein, reviewing recent data investigating its structure and function. We conclude by asking: what remains unknown about this enigmatic viral protein?
Keywords: HIV; Rev; human; immunodeficiency; virus.
Conflict of interest statement
We declare we have no competing interests.
Figures
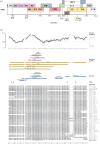
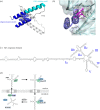

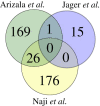
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
