Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA
- PMID: 33352107
- PMCID: PMC7935664
- DOI: 10.1016/j.ymthe.2020.11.011
Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA
Abstract
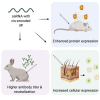
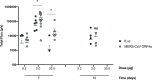
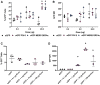
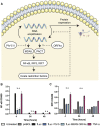
Self-amplifying RNA (saRNA) is a cutting-edge platform for both nucleic acid vaccines and therapeutics. saRNA is self-adjuvanting, as it activates types I and III interferon (IFN), which enhances the immunogenicity of RNA vaccines but can also lead to inhibition of translation. In this study, we screened a library of saRNA constructs with cis-encoded innate inhibiting proteins (IIPs) and determined the effect on protein expression and immunogenicity. We observed that the PIV-5 V and Middle East respiratory syndrome coronavirus (MERS-CoV) ORF4a proteins enhance protein expression 100- to 500-fold in vitro in IFN-competent HeLa and MRC5 cells. We found that the MERS-CoV ORF4a protein partially abates dose nonlinearity in vivo, and that ruxolitinib, a potent Janus kinase (JAK)/signal transducer and activator of transcription (STAT) inhibitor, but not the IIPs, enhances protein expression of saRNA in vivo. Both the PIV-5 V and MERS-CoV ORF4a proteins were found to enhance the percentage of resident cells in human skin explants expressing saRNA and completely rescued dose nonlinearity of saRNA. Finally, we observed that the MERS-CoV ORF4a increased the rabies virus (RABV)-specific immunoglobulin G (IgG) titer and neutralization half-maximal inhibitory concentration (IC50) by ∼10-fold in rabbits, but not in mice or rats. These experiments provide a proof of concept that IIPs can be directly encoded into saRNA vectors and effectively abate the nonlinear dose dependency and enhance immunogenicity.
Keywords: RNA; gene delivery; immunomodulation; innate immunity; interferon; nanoparticles; replicon; self-amplifying; vaccines.
Crown Copyright © 2020. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.K.B., P.F.M., and R.J.S. are co-inventors of a patent resulting from this work. The remaining authors declare no competing interests.
Figures



References
-
- Perri S., Greer C.E., Thudium K., Doe B., Legg H., Liu H., Romero R.E., Tang Z., Bin Q., Dubensky T.W., Jr. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003;77:10394–10403. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

