DNA vaccines against COVID-19: Perspectives and challenges
- PMID: 33352173
- PMCID: PMC7749647
- DOI: 10.1016/j.lfs.2020.118919
DNA vaccines against COVID-19: Perspectives and challenges
Abstract
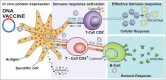
The coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is associated with several fatal cases worldwide. The rapid spread of this pathogen and the increasing number of cases highlight the urgent development of vaccines. Among the technologies available for vaccine development, DNA vaccination is a promising alternative to conventional vaccines. Since its discovery in the 1990s, it has been of great interest because of its ability to elicit both humoral and cellular immune responses while showing relevant advantages regarding producibility, stability, and storage. This review aimed to summarize the current knowledge and advancements on DNA vaccines against COVID-19, particularly those in clinical trials.
Keywords: COVID-19; Coronavirus; DNA vaccine; Immunization; Nucleic acid-based vaccines.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

References
-
- WHO . 11 March 2020. Director-General’s Opening Remarks at the Media Briefing on COVID-19.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

