Robust scan synchronized force-fluorescence imaging
- PMID: 33352414
- PMCID: PMC8761069
- DOI: 10.1016/j.ultramic.2020.113165
Robust scan synchronized force-fluorescence imaging
Abstract
Simultaneous atomic force microscope (AFM) and sample scanning confocal fluorescence microscope measurements are widely used to obtain mechanistic and structural insights into protein dynamics in live cells. However, the absence of a robust technique to synchronously scan both AFM and confocal microscope piezo stages makes it difficult to visualize force-induced changes in fluorescent protein distribution in cells. To address this challenge, we have built an integrated AFM-confocal fluorescence microscope platform that implements a synchronous scanning method which eliminates image artifacts from piezo motion ramping, produces accurate pixel binning and enables the collection of a scanned image of a sample while applying force to a single point on the sample. As proof of principle, we use this instrument to monitor the redistribution of fluorescent E-cadherin, an essential transmembrane protein, in live cells, upon application of mechanical force.
Keywords: AFM-sample scanning confocal microscope; Integrated AFM–fluorescence microscope; Point scanning; Simultaneous force-fluorescence measurements; Synchronized scanning.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
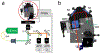


References
-
- Gumpp H et al. , Triggering Enzymatic Activity with Force. Nano Lett. 9, 3290–3295 (2009). - PubMed
-
- Kassies R et al. , Combined AFM and confocal fluorescence microscope for applications in bio-nanotechnology. Journal of microscopy 217, 109–116 (2005). - PubMed
-
- Meller K, Theiss C, Atomic force microscopy and confocal laser scanning microscopy on the cytoskeleton of permeabilised and embedded cells. Ultramicroscopy 106, 320–325 (2006). - PubMed
-
- Noy A, Huser TR, Combined force and photonic probe microscope with single molecule sensitivity. Rev. Sci. Instrum 74, 1217–1221 (2003).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

