Phenolic Constituents of Lamium album L. subsp. album Flowers: Anatomical, Histochemical, and Phytochemical Study
- PMID: 33352709
- PMCID: PMC7766379
- DOI: 10.3390/molecules25246025
Phenolic Constituents of Lamium album L. subsp. album Flowers: Anatomical, Histochemical, and Phytochemical Study
Abstract
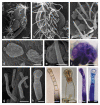
Flos Lamii albi has a high biological activity and is widely used in herbal medicine. The aim of the study was to characterize the secretory structures present in Lamium album subsp. album corolla and the location of phenolic compounds. Additionally, we carried out qualitative phytochemical analyses of flavonoids and phenolic acids. Light, fluorescence, and scanning electron microscopy were used to analyze the structure of the floral organs. The main classes of phenolic compounds and their localization were determined histochemically. Phytochemical analyses were performed with high-performance thin-layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC). Six types of glandular trichomes were found which contained flavonoids, phenolic acids, and tannins. The phytochemical studies demonstrated the presence of caffeic, chlorogenic, ferulic, gallic, p-coumaric, protocatechuic, syringic, gentisic, and vanillic phenolic acids as well as rutoside, isoquercetin, and quercetin flavonoids. The corolla in L. album subsp. album has antioxidant properties due to the presence of various polyphenols, as shown by the histo- and phytochemical analyses. The distribution and morphology of trichomes and the content of phenolic compounds in the corolla have taxonomic, pharmacognostic, and practical importance, facilitating the identification of the raw material.
Keywords: flavonoids; phenolic acids; secretory trichomes; tannins; white nettle corolla.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Mabberley D.J. The Plant Book. 2nd ed. Cambridge University Press; Cambridge, UK: 1997.
-
- Yordanova Z.P., Zhiponova M.K., Iakimova E.T., Dimitrova M.A., Kapchina-Toteva V.M. Revealing the reviving secret of the white dead nettle (Lamium album L.) Phytochem. Rev. 2014;13:375–389. doi: 10.1007/s11101-014-9356-2. - DOI
-
- Dengler J., Berg C., Eisenberg M., Isermann M., Jansen F., Koska I., Löbel S., Manthey M., Pazolt J., Spangenberg A., et al. New descriptions and typifications of syntaxa within the project ‘Plant communities of Mecklenburg-Vorpommern and their vulnerability’—Part I. Feddes Repertorium Zeitschrift für Botanische Taxonomie und Geobotanik. 2003;114:587–631. doi: 10.1002/fedr.200311017. - DOI
-
- Metcalfe C.R., Chalk L. Anatomy of the Dicotyledons. Volume 2 Oxford Press; London, UK: 1972.
-
- Sulborska A., Dmitruk M., Konarska A., Weryszko-Chmielewska E. Adaptations of Lamium album L. flowers to pollination by Apoidea. Acta Sci. Pol. Hortorum Cultus. 2014;13:31–43.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

