Environmental Risk Assessment for rVSVΔG-ZEBOV-GP, a Genetically Modified Live Vaccine for Ebola Virus Disease
- PMID: 33352786
- PMCID: PMC7767225
- DOI: 10.3390/vaccines8040779
Environmental Risk Assessment for rVSVΔG-ZEBOV-GP, a Genetically Modified Live Vaccine for Ebola Virus Disease
Abstract
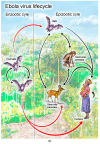
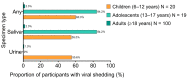
rVSVΔG-ZEBOV-GP is a live, attenuated, recombinant vesicular stomatitis virus (rVSV)-based vaccine for the prevention of Ebola virus disease caused by Zaire ebolavirus. As a replication-competent genetically modified organism, rVSVΔG-ZEBOV-GP underwent various environmental evaluations prior to approval, the most in-depth being the environmental risk assessment (ERA) required by the European Medicines Agency. This ERA, as well as the underlying methodology used to arrive at a sound conclusion about the environmental risks of rVSVΔG-ZEBOV-GP, are described in this review. Clinical data from vaccinated adults demonstrated only infrequent, low-level shedding and transient, low-level viremia, indicating a low person-to-person infection risk. Animal data suggest that it is highly unlikely that vaccinated individuals would infect animals with recombinant virus vaccine or that rVSVΔG-ZEBOV-GP would spread within animal populations. Preclinical studies in various hematophagous insect vectors showed that these species were unable to transmit rVSVΔG-ZEBOV-GP. Pathogenicity risk in humans and animals was found to be low, based on clinical and preclinical data. The overall risk for non-vaccinated individuals and the environment is thus negligible and can be minimized further through defined mitigation strategies. This ERA and the experience gained are relevant to developing other rVSV-based vaccines, including candidates under investigation for prevention of COVID-19.
Keywords: ERA; ERVEBO®; GMO; Zaire ebolavirus; environmental impact; rVSV; recombinant vaccine; shedding; vesicular stomatitis virus; viremia.
Conflict of interest statement
J.G.T., B-A.G.C., S.A.D., W.L., L.W., and J.W. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA. U.J. served as a consultant to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, in connection with the work described in this manuscript. Employees of the funder were involved in the design of the original research summarized in this article; in the collection, analyses and interpretation of data; and in the writing of the manuscript. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


References
-
- Canady D., Weil N.C., Miller C., Shantha J.G., Bastien G., Yeh S. Ophthalmic and Psychosocial Sequelae in Ebola Virus Disease Survivors: Ongoing Need for Health Systems Strengthening across Disciplines. Expert Rev. Anti. Infect. Ther. 2020:1–3. doi: 10.1080/14787210.2020.1808461. - DOI - PMC - PubMed
-
- Etard J.F., Sow M.S., Leroy S., Toure A., Taverne B., Keita A.K., Msellati P., Magassouba N., Baize S., Raoul H., et al. Multidisciplinary Assessment of Post-Ebola Sequelae in Guinea (Postebogui): An Observational Cohort Study. Lancet Infect. Dis. 2017;17:545–552. doi: 10.1016/S1473-3099(16)30516-3. - DOI - PubMed
-
- Mattia J.G., Vandy M.J., Chang J.C., Platt D.E., Dierberg K., Bausch D.G., Brooks T., Conteh S., Crozier I., Fowler R.A., et al. Early Clinical Sequelae of Ebola Virus Disease in Sierra Leone: A Cross-Sectional Study. Lancet Infect. Dis. 2016;16:331–338. doi: 10.1016/S1473-3099(15)00489-2. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

