Macrophage Proinflammatory Responses to Microorganisms and Transplanted Organs
- PMID: 33352942
- PMCID: PMC7766629
- DOI: 10.3390/ijms21249669
Macrophage Proinflammatory Responses to Microorganisms and Transplanted Organs
Abstract
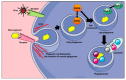
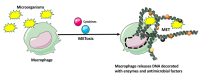
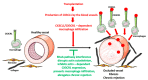
Tissue-resident macrophages and those conscripted from the blood/bone marrow are professional phagocytes. They play a role in tissue homeostasis, replacement, and healing, and are the first-line responders to microbial (viral, bacterial, and fungi) infections. Intrinsic ameboid-type motility allows non-resident macrophages to move to the site of inflammation or injury, where, in response to the inflammatory milieu they perform the anti-microbial and/or tissue repair functions. Depending on the need and the signaling from the surrounding tissue and other immune cells, macrophages acquire morphologically and functionally different phenotypes, which allow them to play either pro-inflammatory or anti-inflammatory functions. As such, the macrophages are also the major players in the rejection of the transplanted organs making an excellent target for the novel anti-rejection therapies in clinical transplantation. In this review, we describe some of the less covered aspects of macrophage response to microbial infection and organ transplantation.
Keywords: chronic rejection; infection; macrophage; transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Liao X., Shen Y., Zhang R., Sugi K., Vasudevan N.T., Alaiti M.A., Sweet D.R., Zhou L., Qing Y., Gerson S.L., et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2018;115:E4661–E4669. doi: 10.1073/pnas.1720065115. - DOI - PMC - PubMed
-
- Nagelkerke S.Q., Bruggeman C.W., Haan J.M.D., Mul E.P.J., Berg T.K.V.D., Van Bruggen R., Kuijpers T.W. Red pulp macrophages in the human spleen are a distinct cell population with a unique expression of Fc-γ receptors. Blood Adv. 2018;2:941–953. doi: 10.1182/bloodadvances.2017015008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

