Antimicrobial Constituents from Machaerium Pers.: Inhibitory Activities and Synergism of Machaeriols and Machaeridiols against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus faecium, and Permeabilized Gram-Negative Pathogens
- PMID: 33352963
- PMCID: PMC7765828
- DOI: 10.3390/molecules25246000
Antimicrobial Constituents from Machaerium Pers.: Inhibitory Activities and Synergism of Machaeriols and Machaeridiols against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus faecium, and Permeabilized Gram-Negative Pathogens
Abstract
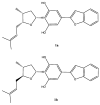
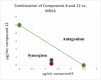
Two new epimeric bibenzylated monoterpenes machaerifurogerol (1a) and 5-epi-machaerifurogerol (1b), and four known isoflavonoids (+)-vestitol (2), 7-O-methylvestitol (3), (+)-medicarpin (4), and 3,8-dihydroxy-9-methoxypterocarpan (5) were isolated from Machaerium Pers. This plant was previously assigned as Machaerium multiflorum Spruce, from which machaeriols A-D (6-9) and machaeridiols A-C (10-12) were reported, and all were then re-isolated, except the minor compound 9, for a comprehensive antimicrobial activity evaluation. Structures of the isolated compounds were determined by full NMR and mass spectroscopic data. Among the isolated compounds, the mixture 10 + 11 was the most active with an MIC value of 1.25 μg/mL against methicillin-resistant Staphylococcus aureus (MRSA) strains BAA 1696, -1708, -1717, -33591, and vancomycin-resistant Enterococcus faecium (VRE 700221) and E. faecalis (VRE 51299) and vancomycin-sensitive E. faecalis (VSE 29212). Compounds 6-8 and 10-12 were found to be more potent against MRSA 1708, and 6, 11, and 12 against VRE 700221, than the drug control ciprofloxacin and vancomycin. A combination study using an in vitro Checkerboard method was carried out for machaeriols (7 or 8) and machaeridiols (11 or 12), which exhibited a strong synergistic activity of 12 + 8 (MIC 0.156 and 0.625 µg/mL), with >32- and >8-fold reduction of MIC's, compared to 12, against MRSA 1708 and -1717, respectively. In the presence of sub-inhibitory concentrations on polymyxin B nonapeptide (PMBN), compounds 10 + 11, 11, 12, and 8 showed activity in the range of 0.5-8 µg/mL for two strains of Acinetobacter baumannii, 2-16 µg/mL against Pseudomonas aeruginosa PAO1, and 2 µg/mL against Escherichia coli NCTC 12923, but were inactive (MIC > 64 µg/mL) against the two isolates of Klebsiella pneumoniae.
Keywords: 5-epi-machaerifurogerol; MRSA; Machaerium Rimachi 12161; VRE; gram negative bacteria; isoflavonoid; machaeridiol A–C; machaerifurogerol; machaeriol A–C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mabberley D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses. Cambridge University Press; Cambridge, UK: 2017.
-
- Polhill R.M., Raven P.H. Advances in Legume Systematics, Part 1. Royal Botanic Gardens; Kew, UK: 1981.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

