Recent Studies on Supercapacitors with Next-Generation Structures
- PMID: 33353019
- PMCID: PMC7767088
- DOI: 10.3390/mi11121125
Recent Studies on Supercapacitors with Next-Generation Structures
Abstract
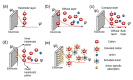
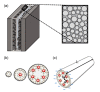
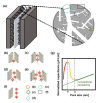
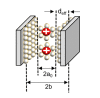
Supercapacitors have shown great potential as a possible solution to the increasing global demand for next-generation energy storage systems. Charge repositioning is based on physical or chemical mechanisms. There are three types of supercapacitors-the electrochemical double layer, the pseudocapacitor, and a hybrid of both. Each type is further subdivided according to the material used. Herein, a detailed overview of the working mechanism as well as a new method for capacitance enhancement are presented.
Keywords: EDLC; Helmholtz model; electrical double layer capacitor; energy storage system; micropores; nanopores; negative capacitance; pseudocapacitor; supercapacitor; ultracapacitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ko E., Lee J.W., Shin C. Negative Capacitance FinFET With Sub-20-mV/decade Subthreshold Slope and Minimal Hysteresis of 0.48 V. IEEE Electron Device Lett. 2017;38:418–421. doi: 10.1109/LED.2017.2672967. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

