Expression of a Hyperthermophilic Cellobiohydrolase in Transgenic Nicotiana tabacum by Protein Storage Vacuole Targeting
- PMID: 33353085
- PMCID: PMC7767180
- DOI: 10.3390/plants9121799
Expression of a Hyperthermophilic Cellobiohydrolase in Transgenic Nicotiana tabacum by Protein Storage Vacuole Targeting
Abstract
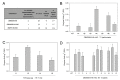
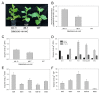
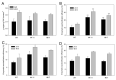
Plant expression of microbial Cell Wall Degrading Enzymes (CWDEs) is a valuable strategy to produce industrial enzymes at affordable cost. Unfortunately, the constitutive expression of CWDEs may affect plant fitness to variable extents, including developmental alterations, sterility and even lethality. In order to explore novel strategies for expressing CWDEs in crops, the cellobiohydrolase CBM3GH5, from the hyperthermophilic bacterium Caldicellulosiruptor saccharolyticus, was constitutively expressed in N. tabacum by targeting the enzyme both to the apoplast and to the protein storage vacuole. The apoplast targeting failed to isolate plants expressing the recombinant enzyme despite a large number of transformants being screened. On the opposite side, the targeting of the cellobiohydrolase to the protein storage vacuole led to several transgenic lines expressing CBM3GH5, with an enzyme yield of up to 0.08 mg g DW-1 (1.67 Units g DW-1) in the mature leaf tissue. The analysis of CBM3GH5 activity revealed that the enzyme accumulated in different plant organs in a developmental-dependent manner, with the highest abundance in mature leaves and roots, followed by seeds, stems and leaf ribs. Notably, both leaves and stems from transgenic plants were characterized by an improved temperature-dependent saccharification profile.
Keywords: hyperthermophilic cellobiohydrolase; plant cell wall; plant immunity; protein storage vacuole; saccharification; transgenic Nicotiana tabacum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Benedetti M., Locci F., Gramegna G., Sestili F., Savatin D.-V. Green production and biotechnological applications of cell wall lytic enzymes. Appl. Sci. 2019;9:5012. doi: 10.3390/app9235012. - DOI
-
- Boudart G., Charpentier M., Lafitte C., Martinez Y., Jauneau A., Gaulin E., Esquerré-Tugayé M.-T., Dumas B. Elicitor activity of a fungal endopolygalacturonase in tobacco requires a functional catalytic site and cell wall localization. Plant Physiol. 2003 doi: 10.1104/pp.011585. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

