Skeletal Muscle Mitochondrial Dysfunction and Oxidative Stress in Peripheral Arterial Disease: A Unifying Mechanism and Therapeutic Target
- PMID: 33353218
- PMCID: PMC7766400
- DOI: 10.3390/antiox9121304
Skeletal Muscle Mitochondrial Dysfunction and Oxidative Stress in Peripheral Arterial Disease: A Unifying Mechanism and Therapeutic Target
Abstract
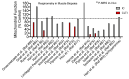
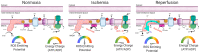
Peripheral artery disease (PAD) is caused by atherosclerosis in the lower extremities, which leads to a spectrum of life-altering symptomatology, including claudication, ischemic rest pain, and gangrene requiring limb amputation. Current treatments for PAD are focused primarily on re-establishing blood flow to the ischemic tissue, implying that blood flow is the decisive factor that determines whether or not the tissue survives. Unfortunately, failure rates of endovascular and revascularization procedures remain unacceptably high and numerous cell- and gene-based vascular therapies have failed to demonstrate efficacy in clinical trials. The low success of vascular-focused therapies implies that non-vascular tissues, such as skeletal muscle and oxidative stress, may substantially contribute to PAD pathobiology. Clues toward the importance of skeletal muscle in PAD pathobiology stem from clinical observations that muscle function is a strong predictor of mortality. Mitochondrial impairments in muscle have been documented in PAD patients, although its potential role in clinical pathology is incompletely understood. In this review, we discuss the underlying mechanisms causing mitochondrial dysfunction in ischemic skeletal muscle, including causal evidence in rodent studies, and highlight emerging mitochondrial-targeted therapies that have potential to improve PAD outcomes. Particularly, we will analyze literature data on reactive oxygen species production and potential counteracting endogenous and exogenous antioxidants.
Keywords: bioenergetics; ischemia; myopathy; peripheral vascular disease; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fowkes F., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., Norman P.E., Sampson U.K.A., Williams L.J., Mensah G.A., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. - DOI - PubMed
-
- Tsai T.T., Rehring T.F., Rogers R.K., Shetterly S.M., Wagner N.M., Gupta R., Jazaeri O., Hedayati N., Jones W.S., Patel M.R., et al. The Contemporary Safety and Effectiveness of Lower Extremity Bypass Surgery and Peripheral Endovascular Interventions in the Treatment of Symptomatic Peripheral Arterial Disease. Circilation. 2015;132:1999–2011. doi: 10.1161/CIRCULATIONAHA.114.013440. - DOI - PMC - PubMed
-
- Taylor S.M., Kalbaugh C.A., Blackhurst D.W., Cass A.L., Trent E.A., Langan E.M., Youkey J.R. Determinants of functional outcome after revascularization for critical limb ischemia: An analysis of 1000 consecutive vascular interventions. J. Vasc. Surg. 2006;44:747–756. doi: 10.1016/j.jvs.2006.06.015. - DOI - PubMed
-
- Rajagopalan S., Mohler E.R., Lederman R.J., Mendelsohn F.O., Saucedo J.F., Goldman C.K., Blebea J., Macko J., Kessler P.D., Rasmussen H.S., et al. Regional Angiogenesis With Vascular Endothelial Growth Factor in Peripheral Arterial Disease. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. - DOI - PubMed
Publication types
Grants and funding
- R01 HL149704/HL/NHLBI NIH HHS/United States
- T32 GM008721/GM/NIGMS NIH HHS/United States
- R01-HL149704/National Institutes of Health; National Heart, Lung, and Blood Institute (NHLBI)
- 18CDA34110044/American Heart Association
- R01-HL148597/National Institutes of Health; National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources

