How Beat Perception Co-opts Motor Neurophysiology
- PMID: 33353800
- PMCID: PMC9440376
- DOI: 10.1016/j.tics.2020.11.002
How Beat Perception Co-opts Motor Neurophysiology
Abstract
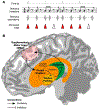
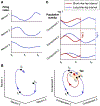
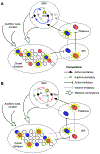
Beat perception offers cognitive scientists an exciting opportunity to explore how cognition and action are intertwined in the brain even in the absence of movement. Many believe the motor system predicts the timing of beats, yet current models of beat perception do not specify how this is neurally implemented. Drawing on recent insights into the neurocomputational properties of the motor system, we propose that beat anticipation relies on action-like processes consisting of precisely patterned neural time-keeping activity in the supplementary motor area (SMA), orchestrated and sequenced by activity in the dorsal striatum. In addition to synthesizing recent advances in cognitive science and motor neuroscience, our framework provides testable predictions to guide future work.
Keywords: basal ganglia; beat perception; motor system; music cognition; supplementary motor area.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- Nettl B (2015) The Study of Ethnomusicology: Thirty-Three Discussions, University of Illinois Press.
-
- Damm L et al. (2020) Why do we move to the beat? A multi-scale approach, from physical principles to brain dynamics. Neuroscience & Biobehavioral Reviews 112, 553–584 - PubMed
-
- Manning F and Schutz M (2013) “Moving to the beat” improves timing perception. Psychonomic Bulletin & Review 20, 1133–1139 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

