Lyme Disease Pathogenesis
- PMID: 33353871
- PMCID: PMC8046170
- DOI: 10.21775/cimb.042.473
Lyme Disease Pathogenesis
Abstract
Lyme disease Borrelia are obligately parasitic, tick- transmitted, invasive, persistent bacterial pathogens that cause disease in humans and non-reservoir vertebrates primarily through the induction of inflammation. During transmission from the infected tick, the bacteria undergo significant changes in gene expression, resulting in adaptation to the mammalian environment. The organisms multiply and spread locally and induce inflammatory responses that, in humans, result in clinical signs and symptoms. Borrelia virulence involves a multiplicity of mechanisms for dissemination and colonization of multiple tissues and evasion of host immune responses. Most of the tissue damage, which is seen in non-reservoir hosts, appears to result from host inflammatory reactions, despite the low numbers of bacteria in affected sites. This host response to the Lyme disease Borrelia can cause neurologic, cardiovascular, arthritic, and dermatologic manifestations during the disseminated and persistent stages of infection. The mechanisms by which a paucity of organisms (in comparison to many other infectious diseases) can cause varied and in some cases profound inflammation and symptoms remains mysterious but are the subjects of diverse ongoing investigations. In this review, we provide an overview of virulence mechanisms and determinants for which roles have been demonstrated in vivo, primarily in mouse models of infection.
Figures


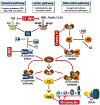



References
-
- Adams PP, Flores Avile C, Popitsch N, Bilusic I, Schroeder R, Lybecker M, and Jewett MW (2017). In vivo expression technology and 5’ end mapping of the Borrelia burgdorferi transcriptome identify novel RNAs expressed during mammalian infection. Nucleic Acids Research 45, 775–792. 10.1093/nar/gkw1180 - DOI - PMC - PubMed
-
- Aguirre JD, Clark HM, McIlvin M, Vazquez C, Palmere SL, Grab DJ, Seshu J, Hart PJ, Saito M, and Culotta VC (2013). A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. The Journal of Biological Chemistry 288, 8468–8478. 10.1074/jbc.M112.433540 - DOI - PMC - PubMed
-
- Alitalo A, Meri T, Lankinen H, Seppala I, Lahdenne P, Hefty PS, Akins D, and Meri S (2002). Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J Immunol 169, 3847–3853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS104767/NS/NINDS NIH HHS/United States
- R01 AI150157/AI/NIAID NIH HHS/United States
- R21 AI133367/AI/NIAID NIH HHS/United States
- R01 AI118799/AI/NIAID NIH HHS/United States
- R01 AI099094/AI/NIAID NIH HHS/United States
- R01 AI131656/AI/NIAID NIH HHS/United States
- R01 AI037277/AI/NIAID NIH HHS/United States
- R21 AI103905/AI/NIAID NIH HHS/United States
- R01 AI059048/AI/NIAID NIH HHS/United States
- K22 AI081730/AI/NIAID NIH HHS/United States
- R56 AI146930/AI/NIAID NIH HHS/United States
- R21 AI111317/AI/NIAID NIH HHS/United States
- R21 AI140510/AI/NIAID NIH HHS/United States
- R01 AI146930/AI/NIAID NIH HHS/United States
- R01 AI121217/AI/NIAID NIH HHS/United States
- R01 AI121401/AI/NIAID NIH HHS/United States
- R21 AI119821/AI/NIAID NIH HHS/United States
- R21 NS102766/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

